Resistive Switching Characteristics of HfO2 Thin Films on Mica Substrates Prepared by Sol-Gel Process
Abstract
:1. Introduction
2. Materials and Methods
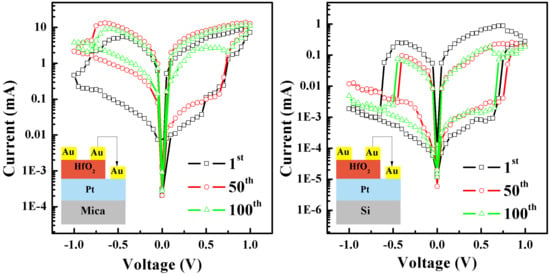
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lim, E.W.; Ismail, R. Conduction Mechanism of Valence Change Resistive Switching Memory A Survey. Electronics 2015, 4, 586–613. [Google Scholar] [CrossRef]
- Lin, C.A.; Huang, C.J.; Tseng, T.Y. Impact of barrier layer on HfO2-based conductive bridge random access memory. Appl. Phys. Lett. 2019, 114, 093105. [Google Scholar] [CrossRef]
- Han, P.D.; Sun, B.; Cheng, S.; Yu, F.Y. An optoelectronic resistive switching memory behavior of Ag/α-SnWO4/FTO device. J. Alloy Compd. 2016, 681, 516–521. [Google Scholar] [CrossRef]
- Wei, X.D.; Huang, H.; Ye, C.; Wei, W.; Zhou, H.; Chen, Y.; Zhang, R.L.; Zhang, L.; Xia, Q. Exploring the role of nitrogen incorporation in ZrO2 resistive switching film for enhancing the device performance. J. Alloy. Compd. 2019, 775, 1301–1306. [Google Scholar] [CrossRef]
- Hsu, C.C.; Wang, T.C.; Tsao, C.C. Forming-free sol-gel ZrOx resistive switching memory. J. Alloy. Compd. 2018, 769, 65–70. [Google Scholar] [CrossRef]
- Cheng, T.D.; Zhang, H.; Liu, N.; Yu, P.F.; Wu, C.T.; Tang, X.G. Improvement of memristive properties in CuO films with a seed Cu layer. Appl. Phys. Lett. 2019, 114, 061602. [Google Scholar]
- Dai, Y.H.; Zhao, Y.Y.; Wang, J.Y.; Xu, J.B.; Yang, F. First principle simulations on the effects of oxygen vacancy in HfO2-based RRAM. AIP Adv. 2015, 5, 017133. [Google Scholar] [CrossRef]
- Xue, K.H.; Miao, X.S. Oxygen vacancy chain and conductive filament formation in hafnia. J. Appl. Phys. 2018, 123, 16150. [Google Scholar] [CrossRef]
- Chen, C.; Pan, F.; Wang, Z.S.; Yang, J.; Zeng, F. Bipolar resistive switching with self-rectifying effects in Al/ZnO/Si structure. Appl. Phys. Lett. 2012, 111, 013702. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Long, S.B.; Li, Y.; Liu, Q.; Lv, H.B.; Miranda, E.A.; Sune, J.D.; Liu, M. Analysis on the Filament Structure Evolution in Reset Transition of Cu/HfO2/Pt RRAM Device. Nanoscale. Res. Lett. 2016, 11, 269. [Google Scholar] [CrossRef]
- Chand, U.; Huang, K.C.; Huang, C.Y.; Tseng, T.Y. Mechanism of Nonlinear Switching in HfO2-Based Crossbar RRAM With Inserting Large Bandgap Tunneling Barrier Layer. IEEE Trans. Electron Dev. 2015, 62, 3665–3670. [Google Scholar] [CrossRef]
- Gergel-Hackett, N.; Hamadani, B.; Dunlap, B.; Suehle, J.; Richter, C.; Hacker, C.; Gundlach, D. A Flexible Solution-Processed Memristor. IEEE Electron Dev. Lett. 2009, 30, 706–708. [Google Scholar] [CrossRef]
- Zhou, Z.; Mao, H.; Wang, X.; Sun, T.; Chang, Q.; Chen, Y.; Xiu, F.; Liu, Z.; Liu, J.; Huang, W. Transient and flexible polymer memristors utilizing full-solution processed polymer nanocomposites. Nanoscale 2018, 10, 14824–14829. [Google Scholar] [CrossRef] [PubMed]
- Moller, S.; Perlov, C.; Jackson, W.; Taussig, C.; Forrest, S.R. A polymer/semiconductor write-once read-many-times memory. Lett. Nat. 2003, 426, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Figà, V.; Ustab, H.; Macaluso, R.; Salznerd, U.; Ozdemirb, M.; Kulyke, B.; Krupkaf, O.; Bruno, M. Electrochemical polymerization of ambipolar carbonyl-functionalized indenofluorene with memristive properties. Opt. Mater. 2019, 94, 187–195. [Google Scholar] [CrossRef]
- Gutowski, M.; Jaffe, J.E.; Liu, C.L.; Stoker, M.; Hegde, R.I.; Rai, R.S.; Tobin, P.J. Thermodynamic stability of high-K dielectric metal oxides ZrO2 and HfO2 in contact with Si and SiO2. Appl. Phys. Lett. 2002, 80, 1897. [Google Scholar] [CrossRef]
- Ku, B.; Abbas, Y.; Sokolov, A.S.; Choi, C. Interface engineering of ALD HfO2-based RRAM with Ar plasma treatment for reliable and uniform switching behaviors. J. Alloy Compd. 2017, 735, 1181–1188. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Goux, L.; Clima, S.; Govoreanu, B.; Degraeve, R.; Kar, G.S.; Fantini, A.; Groeseneken, G.; Wouters, D.J.; Jurczak, M. Endurance/retention trade-off on HfO2/metal cap 1T1R bipolar RRAM. IEEE Trans. Electron Dev. 2013, 60, 1114–1121. [Google Scholar] [CrossRef]
- Song, B.; Cao, R.; Xu, H.; Liu, S.; Liu, H.J.; Li, Q.J. A HfO2/SiTe Based Dual-Layer Selector Device with Minor Threshold Voltage Variation. Nanomaterials 2019, 9, 408. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Barua, A.; Bailey, T.; Rush, A.; Kosel, P.; Leedy, K.; Jha, R. Reflection coefficient of HfO2-based RRAM in different resistance states. Appl. Phys. Lett. 2018, 113, 192101. [Google Scholar] [CrossRef]
- Yang, Y.X.; Yuan, G.L.; Yan, Z.B.; Wang, Y.J.; Lu, X.B.; Liu, J.M. Flexible Semitransparent, and Inorganic Resistive Memory based on BaTi0.95Co0.05O3 Film. Adv. Mater. 2017, 29, 1700425. [Google Scholar] [CrossRef]
- Xiao, Z.A.; Zhao, J.H.; Lu, C.; Zhou, Z.Y.; Wang, H.; Zhang, L.; Wang, J.J.; Li, X.Y.; Wang, K.Y.; Zhao, Q.L.; et al. Characteristic investigation of a flexible resistive memory based on a tunneling junction of Pd/BTO/LSMO on mica substrate. Appl. Phys. Lett. 2018, 113, 223501. [Google Scholar] [CrossRef]
- Yang, C.H.; Han, Y.J.; Qian, J.; Lv, P.P.; Lin, X.J.; Huang, S.F.; Cheng, Z.X. Flexible Temperature-Resistant, and Fatigue-Free Ferroelectric Memory Based on Bi(Fe0.93Mn0.05Ti0.02)O3 Thin Film. ACS Appl. Mater. Interfaces 2019, 11, 12647–12655. [Google Scholar] [CrossRef]
- He, G.; Liu, M.; Zhu, L.Q.; Chang, M.; Fang, Q.; Zhang, L.D. Effect of post deposition annealing on the thermal stability and structural characteristics of sputtered HfO2 films on Si (100). Surf. Sci. 2014, 576, 67–75. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, S.B.; Rath, C. Latent Fingerprint Imaging Using Dy and Sm Codoped HfO2 Nanophosphors: Structure and Luminescence Properties. Part. Part. Syst. Charact. 2019, 36, 1900048. [Google Scholar] [CrossRef]
- Bradley, S.R.; Bersuker, G.; Shluger, A.L. Modelling of oxygen vacancy aggregates in monoclinic HfO2: Can they contribute to conductive filament formation? J. Phys. Condens. Mater. 2015, 27, 415401. [Google Scholar] [CrossRef]
- Crist, B.V. Handbook of Monochromatic XPS Spectra, Semiconductors. IEEE Electr. Insul. M. 2003, 19, 47. [Google Scholar]
- Yoon, J.H.; Song, S.J.; Yoo, I.; Seok, J.Y.; Yoon, K.J.; Kwon, D.E.; Park, T.H.; Hwang, C.S. Highly Uniform, Electroforming-Free, and Self-Rectifying Resistive Memory in the Pt/Ta2O5/HfO2−x/TiN Structure. Adv. Funct. Mater. 2014, 24, 5086–5095. [Google Scholar] [CrossRef]
- Ma, H.L.; Zhang, X.M.; Wu, F.C.; Luo, Q.; Gong, T.C.; Yuan, P.; Xu, X.X.; Liu, Y.; Zhao, S.J.; Zhang, K.P.; et al. Self-Rectifying Resistive Switching Device Based on HfO2/TaOx Bilayer Structure. IEEE Trans. Electron Dev. 2019, 66, 924–928. [Google Scholar] [CrossRef]
- Januar, M.; Prakoso, S.P.; Lan, S.Y.; Mahanty, R.K.; Kuo, S.Y.; Liu, K.C. The role of oxygen plasma in the formation of oxygen defects in HfOx films deposited at room temperature. J. Mater. Chem. C 2015, 3, 4104–4114. [Google Scholar] [CrossRef]
- Sokolov, A.S.; Jeon, Y.R.; Kim, S.; Ku, B.; Lim, D.; Han, H.; Chae, M.G.; Lee, J.; Ha, B.G.; Choi, C. Influence of oxygen vacancies in ALD HfO2−x thin films on non-volatile resistive switching phenomena with a Ti/HfO2−x/Pt structure. Appl. Surf. Sci. 2018, 434, 822–830. [Google Scholar] [CrossRef]
- Traore, B.; Blaise, P.; Vianello, E.; Grampeix, H.; Jeannot, S.; Perniola, L.; De Salvo, B.; Nishi, Y. On the Origin of Low-Resistance State Retention Failure in HfO2-Based RRAM and Impact of Doping/Alloying. IEEE Trans. Electron Dev. 2015, 62, 4029–4036. [Google Scholar] [CrossRef]
- Januar, M.; Prakoso, S.P.; Lan, S.Y.; Mahanty, R.K.; Kuo, S.Y.; Liu, K.C. Metal oxide resistive memory switching mechanism based on conductive filament properties. J. Appl. Phys. 2011, 110, 124518. [Google Scholar]
- Padovani, A.; Larcher, L.; Pirrotta, O.; Vandelli, L.; Bersuker, G. Microscopic Modeling of HfOx RRAM Operations: From Forming to Switching. IEEE Trans. Electron Dev. 2015, 62, 1998–2006. [Google Scholar] [CrossRef]
- Chen, P.H.; Su, Y.T.; Chang, F.C. Stabilizing Resistive Switching Characteristics by Inserting Indium-Tin-Oxide Layer as Oxygen Ion Reservoir in HfO2-Based Resistive Random Access Memory. IEEE Trans. Electron Dev. 2019, 66, 1276–1280. [Google Scholar] [CrossRef]
- Qi, M.; Tao, Y.; Wang, Z.Q.; Xu, H.Y.; Zhao, X.N.; Liu, W.Z.; Ma, J.G.; Liu, Y.C. Highly uniform switching of HfO2−x based RRAM achieved through Ar plasma treatment for low power and multilevel storage. Appl. Surf. Sci. 2018, 458, 216–221. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-F.; Tang, X.-G.; Wang, L.-Q.; Tang, H.; Jiang, Y.-P.; Liu, Q.-X.; Li, W.-H.; Tang, Z.-H. Resistive Switching Characteristics of HfO2 Thin Films on Mica Substrates Prepared by Sol-Gel Process. Nanomaterials 2019, 9, 1124. https://doi.org/10.3390/nano9081124
Liu C-F, Tang X-G, Wang L-Q, Tang H, Jiang Y-P, Liu Q-X, Li W-H, Tang Z-H. Resistive Switching Characteristics of HfO2 Thin Films on Mica Substrates Prepared by Sol-Gel Process. Nanomaterials. 2019; 9(8):1124. https://doi.org/10.3390/nano9081124
Chicago/Turabian StyleLiu, Chao-Feng, Xin-Gui Tang, Lun-Quan Wang, Hui Tang, Yan-Ping Jiang, Qiu-Xiang Liu, Wen-Hua Li, and Zhen-Hua Tang. 2019. "Resistive Switching Characteristics of HfO2 Thin Films on Mica Substrates Prepared by Sol-Gel Process" Nanomaterials 9, no. 8: 1124. https://doi.org/10.3390/nano9081124