Silver Nanoparticles with Liquid Crystalline Ligands Based on Lactic Acid Derivatives
Abstract
:1. Introduction
2. Synthesis of Ligand and the Silver Nanoparticle Preparation
3. Experimental Techniques
4. Results and discussion
4.1. Nanoparticle Observation
4.2. Dynamic Light Scattering (DLS) Measurements
4.3. Plasmonic Resonance
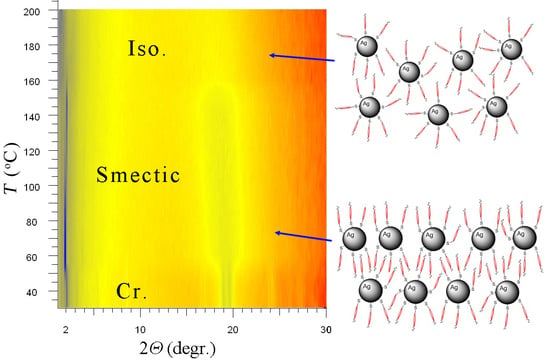
4.4. Mesomorphic Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lewandowski, W.; Lojewska, T.; Szustakiewicz, P.; Mieczkowski, J.; Pociecha, D. Reversible switching of structural and plasmonic properties of liquid-crystalline gold nanoparticle assemblies. Nanoscale 2016, 8, 2656–2663. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Lin Zhao, L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Evanoff, D.D., Jr.; Chumanov, G. Synthesis and Optical Properties of Silver Nanoparticles and Arrays. ChemPhysChem Minirev. 2005, 6, 1221–1231. [Google Scholar] [CrossRef]
- Doyle, W.T. Optical properties of a suspension of metal spheres. Phys. Rev. B 1989, 39, 9852. [Google Scholar] [CrossRef]
- Shang, Z.; Huang, H.; Wan, Y.; Deng, L. Modulation of localized surface plasmon resonance for an array of Ag nanostructures layered with nematic liquid crystals. Opt. Commun. 2016, 372, 80–84. [Google Scholar] [CrossRef]
- Peng, S.; McMahon, J.M.; Schatz, G.C.; Gray, S.K.; Sun, Y. Reversing the size-dependence of surface plasmon resonances. Proc. Natl. Acad. Sci. USA 2010, 107, 14530–14534. [Google Scholar] [CrossRef] [Green Version]
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef]
- Goesmann, H.; Feldmann, C. Nanoparticle functional materials. Angew. Chem. Int. Ed. 2010, 49, 1362–1395. [Google Scholar] [CrossRef]
- Zhang, Q.; Large, N.; Nordlander, P.; Wang, H. Porous Au nanoparticles with tunable plasmon resonances and intense field enhancements for single-particle SERS. J. Phys. Chem. Lett. 2014, 5, 370–374. [Google Scholar] [CrossRef]
- Chakraborty, I.; Bag, S.; Landman, U.; Pradeep, T. Atomically Precise Silver Clusters as New SERS Substrates. J. Phys. Chem. Lett. 2013, 4, 2769–2773. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef]
- Boles, M.A.; Engel, D.M. Talapin: Self-assembly of colloidal nanocrystals: From intricate structures to functional materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef]
- Lewadowski, W.; Fruhnert, M.; Mieczkowski, J.; Rockstuhl, C.; Gorecka, E. Dynamically self-assembled silver nanoparticles as a thermally tunable metamaterial. Nat. Commun. 2015, 6, 6590. [Google Scholar] [CrossRef] [Green Version]
- Wojcik, M.; Olesinska, M.; Sawczyk, M.; Mieczkowski, J.; Gorecka, E. Controlling the spatial organization of liquid crystalline nanoparticles by composition of the organic grafting layer. Chem. A Eur. J. 2015, 21, 10082–10088. [Google Scholar] [CrossRef]
- Wojcik, M.; Kolpaczynska, M.; Pociecha, D.; Mieczkowski, J.; Gorecka, E. Multidimensional structures made by gold nanoparticles with shape-adaptive grafting layers. Soft Matter 2010, 6, 5397–5400. [Google Scholar] [CrossRef]
- Wojcik, M.; Gora, M.; Mieczkowski, J.; Romiszewski, J.; Gorecka, E.; Pociecha, D. Temperature-controlled liquid crystalline polymorphism of gold nanoparticles. Soft Matter 2011, 7, 10561–10564. [Google Scholar] [CrossRef]
- Lewandowski, W.; Wojcik, M.; Gorecka, E. Metal nanoparticles with liquid-crystalline ligands: Controlling nanoparticle superlattice structure and properties. ChemPhysChem 2014, 15, 1283–1295. [Google Scholar] [CrossRef]
- Lewandowski, W.; Constantin, D.; Walicka, K.; Pociecha, D.; Mieczkowski, J.; Gorecka, E. Smectic mesophases of functionalized silver and gold nanoparticles with anisotropic plasmonic properties. Chem. Commun. 2013, 49, 7845–7847. [Google Scholar] [CrossRef]
- Li, B.; Smilgies, D.-M.; Price, A.D.; Huber, D.L.; Clem, P.G.; Fan, H. Poly(N-isopropylacrylamide) surfactant-functionalized responsive silver nanoparticles and superlattices. ASC Nano 2014, 8, 4799–4804. [Google Scholar] [CrossRef]
- Wolska, J.; Pociecha, D.; Mieczkowski, J.; Gorecka, E. Gold nanoparticles with flexible mesogenic grafting layers. Soft Matter 2013, 9, 3005–3008. [Google Scholar] [CrossRef]
- Lewandowski, W.; Jatczak, K.; Pociecha, D.; Mieczkowski, J. Control of gold Nanoparticle superlattice properties via mesogenic ligand architecture. Langmuir 2013, 29, 3404–3410. [Google Scholar] [CrossRef]
- Cathcart, N.; Kitaev, V. Monodisperse Hexagonal Silver Nanoprisms: Synthesis via Thiolate-Protected Cluster Precursors and Chiral, Ligand-Imprinted Self-Assembly. ACS Nano 2011, 5, 7411–7425. [Google Scholar] [CrossRef]
- Stefaniuk, T.; Wrobel, P.; Gorecka, E.; Szoplik, T. Optimum deposition conditions of ultrasmooth silver nanolayers. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Wolska, J.; Pociecha, D.; Mieczkowski, J.; Gorecka, E. Control of sample alignment mode for hybrid lamellar systems based on gold nanoparticles. Chem. Commun. 2014, 50, 7975–7978. [Google Scholar] [CrossRef]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. Farad. Trans. 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Dadosh, T. Synthesis of uniform silver nanoparticles with a controllable size. Mater. Lett. 2009, 63, 2236–2238. [Google Scholar] [CrossRef]
- Filippo, E.; Serra, A.; Buccolieri, A.; Manno, D. Green synthesis of silver nanoparticles with sucrose and maltose: Morphological and structural characterization. J. Non-Cryst. Solids 2010, 356, 344–350. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering; Courier Dover Publications: Mineola, NY, USA, 2000. [Google Scholar]
- Provencher, S. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, W.; El-Sayed, M.A. On the Univarsal Scaling Behavior of the Distance Decay of Plasmon Coupling in Metal Nanoparticle Pairs: A Plasmon Ruler Equation. Nano Lett. 2007, 7, 2080–2088. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Scalia, G. Liquid Crystals with Nano and Microparticles; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2017; pp. 497–523. [Google Scholar]










| LC-Ligand | AgNPs‘ Diameter | |
|---|---|---|
| NP1 | Thiol A | 4–6 nm |
| NP2 | Thiol B | 3–5 nm |
| NP3 | Thiol C | ~20 nm |
| NP4 | Thiol C | 4–6 nm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troha, T.; Kaspar, M.; Hamplova, V.; Cigl, M.; Havlicek, J.; Pociecha, D.; Novotna, V. Silver Nanoparticles with Liquid Crystalline Ligands Based on Lactic Acid Derivatives. Nanomaterials 2019, 9, 1066. https://doi.org/10.3390/nano9081066
Troha T, Kaspar M, Hamplova V, Cigl M, Havlicek J, Pociecha D, Novotna V. Silver Nanoparticles with Liquid Crystalline Ligands Based on Lactic Acid Derivatives. Nanomaterials. 2019; 9(8):1066. https://doi.org/10.3390/nano9081066
Chicago/Turabian StyleTroha, Tinkara, Miroslav Kaspar, Vera Hamplova, Martin Cigl, Jaroslav Havlicek, Damian Pociecha, and Vladimira Novotna. 2019. "Silver Nanoparticles with Liquid Crystalline Ligands Based on Lactic Acid Derivatives" Nanomaterials 9, no. 8: 1066. https://doi.org/10.3390/nano9081066