3.1. Material Characterisation
The SSA of GoHAP™ of 10 nm (206 ± 1 m
2/g) is over four times greater than that of GoHAP™ of 40 nm (49 ± 1 m
2/g). Additionally, its skeleton density is nearly 10% lower: 2.87 ± 0.01 versus 3.09 ± 0.01 g/cm
3, respectively (
Table 1). We suggest that the reduced GoHAP™ density is the result of a greater fraction of the disordered surface layer of nano-hydroxyapatites in the material volume. We described the effect of the nanoparticle density decrease as a function of its SSA increase previously for the example of ZrO
2 nanoparticles [
62].
Figure 1 shows X-ray diffraction line profiles for both sizes of GoHAP™. No phases other than hydroxyapatite were detected. Diffraction profiles for GoHAP™ before and after desorption are essentially identical, which indicates that the desorption process did not impact its crystallinity. The sharper peaks of GoHAP-6 (40 nm) demonstrate that the crystallite size of the latter is larger than that of GoHAP-1 (10 nm). Adsorption and desorption cycles also did not affect the Specific Surface Area and Skeletal Density of GoHAP™ nanoparticles (
Table S2).
Figure 2 presents selected SEM images of GoHAP™. Clusters of spherical objects are visible that have an estimated single constituent size of 10–15 nm for the GoHAP-1 (10 nm) powder and approximately 40 nm for the GoHAP-6 (40 nm) powder.
HR TEM images (
Figure 3) obtained by the bright field technique revealed differences in the morphology of GoHAP™. GoHAP-6 (40 nm) has a polyhedron shape, which reflects the hexagonal crystalline structure of hydroxyapatite. TEM images made by the dark field technique enabled the determination of the GoHAP-6 (40 nm) nanoparticle size, and the mean particle size (MPS) based on a fit to the histogram of the log-normal curve is 39 nm (
Figure 4B). This value coincides with the 40 nm calculated using the SSA and density parameters (SMD
BET,
Table 1). Based on the GoHAP™ diffraction line profile analysis [
60,
61], the mean size of the GoHAP-6 (40 nm) crystallites (MCS
XRD) is 62 ± 43 nm. According to the Scherrer formula, crystallites of GoHAP-6 (40 nm) have a length of 54 nm and a width of 34 nm. In the case of GoHAP-1 (10 nm). Due to the strong tendency to agglomerate, it was not possible to determine the GoHAP-1 (10 nm) mean particle size using microscopic imaging. GoHAP-1 (10 nm) crystallites have a length of 19 nm and a width of 5 nm. An analysis of the GoHAP™ diffraction line profile [
60,
61] revealed that the mean size of the crystallite (MCS
XRD) GoHAP-1 (10 nm) was 10 ± 6 nm.
Table 1 presents a summary of the quantitative nanoparticle characterisation. The obtained results confirmed the previously reported particle size and shape differences between the two types of GoHAP™ [
32].
3.2. Examination of Water Adsorption Process
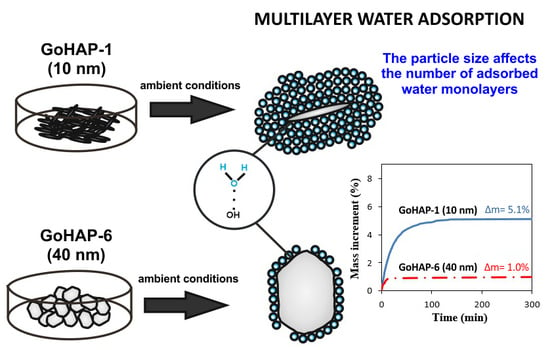
Regardless of the experimental conditions, the kinetic curves of water adsorption from air by GoHAP™ have a similar shape and are composed of three sequential stages: (1) a rapid, nearly linear mass growth; (2) a gradually inhibited process; and (3) the state of equilibrium and stabilised sample mass. The saturated condition is reached no longer than 200 min after the beginning measurement. Over 50% of the equilibrium water mass is adsorbed during the initial 10 min of exposure. The results of adsorption kinetics tests are summarised in
Table 2, and the curves obtained for GoHAP-1 (10 nm) and GoHAP-6 (40 nm) for the ambient relative humidity of 26 ± 2% are presented in
Figure 5. The set of curves recorded for GoHAP-1 (10 nm) at three different experimental conditions (water activity: 0.26, 0.38, and 0.58) are presented in
Figure 5.
With an ambient water activity of 0.26,
Figure 5 shows that the equilibrium increment of the GoHAP-1 (10 nm) mass is five times higher than that of GoHAP-6 (40 nm) (5.2% and 1%, respectively). This result is correlated with the more than fourfold difference between the total surface areas of the tested samples (see
Table 1). The values of the specific adsorption of GoHAP™, in turn, differ to a slight extent and are 0.25 and 0.21 mg/m
2, respectively.
Regarding the impact of the water activity on the kinetics of adsorption, in the case of GoHAP-1 (10 nm), no difference was observed during the initial 10 min of exposure for a relative humidity of 26% and 38%. After that period at the ambient relative humidity of 38%, the mass increase is faster than for a relative humidity of 26% and reaches a state of equilibrium after 70 min. The saturated condition for the relative humidity of 26% is reached after 140 min. Despite differences in the adsorption kinetics, the quantity of water adsorbed by GoHAP-1 (10 nm) at the ambient relative humidity of 26% and 38% is similar and reaches slightly above 5% (
Figure 6). At the ambient relative humidity of 58%, in turn, the water adsorption rate for the two initial stages of the process is considerably faster than at the lower relative humidity. Adsorption at the ambient relative humidity of 58% is 8.2%, e.g., 1.5 times higher than for 26% and 38% relative humidity. In other units for the surface, for a relative humidity of 58%, 0.4 mg of H
2O is bound to 1 m
2 on the GoHAP™ surface, while for 26% and 38% humidity, the value is about 0.25 mg of H
2O/m
2.
The kinetics of water adsorption of GoHAP™ was compared with a reference sample of silica gel. Samples were exposed for 2 h to the ambient relative humidity of 38 ± 2% and 58 ± 2% (
Figure 7).
The silica gel mass did not stabilise within the duration of the experiment, and after two hours of adsorption, it increased by 10–11%. The adsorption rate of the silica gel was the same for both ambient water activity values. The experiment indicated that, at the first stage of the process during the initial 30 min of exposure, GoHAP-1 (10 nm) was characterised by a considerably higher activity compared to silica gel. This result is significant because it shows that GoHAP-1 (10 nm) not only has very good sorption properties, but also can be a more effective and competitive sorbent than silica gel if there is a need to rapidly reduce the relative air humidity.
Based on the results of kinetic tests, an isotherm of water vapour adsorption on GoHAP-1 (10 nm) and GoHAP-6 (40 nm) was determined.
Figure 8 presents the isotherm expressed as a change in the relative increment of GoHAP™ mass as a function of water activity. The experimental curve was compared with the isotherm models described in the relevant literature. It was found that the nature of the adsorption isotherm was consistent with the Brunauer—Emmet—Teller (BET) isotherm model [
63,
64], described by Equation (3):
where:
a is the total volume of adsorbed gas under pressure
p (g H
2O/g GoHAP);
p0 is the pressure of the adsorbate’s saturated vapour (Pa);
am is the volume of the monolayer (g H
2O/g GoHAP);
C is the adsorption equilibrium constant; and
p/
p0 =
aw is the water activity.
The parameters for fitting the BET adsorption isotherm to the experimental data were determined (
Figure 8,
Table 3). However, the equation for this isotherm best fits the low range of pressures.
The GoHAP™ adsorption isotherm is characteristic of type III isotherms according to Brunauer’s classification [
64]. This implies that hydrogen bonds are formed between the adsorbent (GoHAP™) and the adsorbate (water) that are stronger than intermolecular interactions such as van der Waals forces. The isotherm type also suggests that a multilayer adsorption process occurs on the GoHAP™ surface. The parameter am (
Table 3) corresponds to a capacity of one layer of water molecules adsorbed per 1 gram of GoHAP™. Therefore, knowledge of the am value is needed to calculate the number of water layers under given conditions.
The thickness of the water layer adsorbed on a GoHAP‒1 surface was determined after exposure for 20 h to air with a relative humidity of 3%, 24%, 38%, 51%, 62%, and 94% (
Table 4). For GoHAP-6 (40 nm), the thickness of the water layer was revealed for a relative humidity of 3%, 13%, 24%, 30%, 38%, and 97% and an exposure time of 20 h (
Table 5). The monolayer water coverage was calculated both by the SSA method of the HA sample [
29,
31] and by using moisture sorption isotherm data (
Figure 8) fitted to the modified BET equation. The mass of one water molecule is 2.99·10
−23 g, and the cross-sectional area of a water molecule must take into account the packing density between water molecules and the hydroxyapatite surface. The cross-sectional area for the adsorption of a water molecule onto the surface of HAP has previously been reported as 0.115 nm
2 [
29].
The monolayer coating calculated using the modified BET method was lower than that calculated using the surface area approach, similar to calculations [
31] for granulated hydroxyapatite. According to the authors of Reference [
31], the BET approach will probably underestimate the true coverage of the monolayer, and it can be assumed that a specific approach to the surface can provide a more accurate determination of the monolayer coverage. Therefore, the number of water layers was calculated by dividing the humidity sample content by the monolayer water coverage of 0.2605 mg/m
2 [
29]. The calculation included the packing efficiency of 0.74 [
65].
The results of a thermogravimetric analysis coupled with differential scanning calorimetry of GoHAP™ are presented in
Figure 9. The thermogravimetric (TG) curve reflects the changes in the mass of the sample as a function of temperature. The calorimetry curve (DSC) reflects the heat flow corresponding to the processes occurring in the sample. Mass spectrometry results, presenting ion currents of H
2O (m/z = 18) and CO
2 (m/z = 44) in gases released from samples during the TG experiment, are shown in
Figure 8. In addition, derivatives of the thermogravimetric curves (DTG) are drawn in
Figure 10 to allow a comparison between the analysed ion current changes and the mass changes in the samples.
A sample mass decrease was observed throughout the entire TG experiments. During the isothermal stage at room temperature, the nanoparticle surface was cleaned just as a result of helium flushing. A significant acceleration in the mass change occurs after the beginning of the heating stage. The highest speed of mass loss (seen as a peak on the DTG curve), correlating with an increase in water desorption (reflected as a peak on the H
2O line), was recorded after 10 min of heating. Intense water desorption occurred up to approximately 150 °C. This is a 150 °C lower temperature than the desorption temperature of microcrystalline hydroxyapatite proposed by Holmes et al. [
30]. According to Reference [
32], structural water is removed from GoHAP™ in the temperature range 200 °C to 600 °C. In the present work, this phenomenon overlaps with the removal of CO
2 molecules from hydroxyapatite, resulting in a slight increase of the ionic current value (
Figure 10). The sum of both processes was reflected as a small peak on the DTG curve of GoHAP-1 (10 nm) and is more intense on the DSC curves for GoHAP-1 (10 nm) and GoHAP-6 (40 nm) (
Figure 9 and
Figure 10).
Thermal analysis coupled with mass spectroscopy of both hydroxyapatite sizes showed that the recorded mass loss results mostly from dehydration. However, it also clearly revealed significant differences between the rate and effectiveness of water desorption depending on nanoparticle size. While flushing the GoHAP-1 (10 nm) with helium at room temperature for 1 h, the mass loss was 1.64 wt %. The weight loss resulting from heating to 800 °C was 7.59 wt %. In total, the GoHAP-1 (10 nm) mass loss was 9.23 wt %. For GoHAP-6 (40 nm), the helium flush stage resulted in a mass loss of 0.26%, and heating the sample to 800 °C resulted in 1.78% of the mass being lost. In total, the mass of GoHAP-6 (40 nm) decreased by 2.04%. Mass changes during the TG process are similar to those obtained in the desorption station as a result of heating for 2.5 h at 150 °C and flushing with helium (
Table 6). This demonstrates that an appropriate selection of the sample desorption conditions leads to nearly total degassing of GoHAP™ without high temperature treatment.