Graphene Oxide/Ferrocene-Containing Polymer/Gold Nanoparticle Triple Nanocomposite
Abstract
:1. Introduction
2. Experimental
2.1. Characterization
2.2. Preparation of 2-((2-(Methacryloyloxy)ethyl)disulfanyl)ethyl Ferrocene-carboxylate (FcMAss)
2.3. Preparation of PFcMAss via ATRP of FcMAss
2.4. Preparation of PFcMAss-grafted Graphene Oxide Sheets (GO-PFcMAss)
2.5. Fabrication of PFcMAss-AuNPs
2.6. Fabrication of GO-PFcMAss-AuNPs Nanocomposite
3. Results and Discussion
3.1. SynthesisofFcMAss Monomer and Preparation of FcMAss Homopolymer
3.2. Preparation of PFcMAss-Grafted Graphene Sheets (GO-PFcMAss)
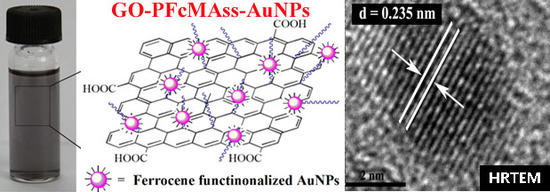
3.3. Fabrication of GO-PFcMAss-AuNPs Nanocomposite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer grapheme. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Viola, W.; Andrew, T.L. High energy density, super-deformable, garment-integrated micro- supercapacitors for powering wearable electronics. ACS Appl. Mater. Interfaces 2018, 10, 36834–36840. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, K.K.; Benayad, A.; Yoon, S.; Park, H.K.; Jung, I.; Jin, M.H.; Jeong, H.; Kim, J.M.; Choi, J.; et al. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Yang, J.; Chhowalla, M.; Loh, K.P. Synthesis and reduction of large sized graphene oxide sheets. Chem. Soc. Rev. 2017, 46, 7306–7316. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J.X. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Pan, Y.Z.; Bao, H.Q.; Sahoo, N.G.; Wu, T.F.; Li, L. Water-soluble poly(N-isopropylacrylamide)-graphene sheets synthesized via click chemistry for drug delivery. Adv. Funct. Mater. 2011, 21, 2754–2763. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Peng, C.; Yu, M.; Li, L.; Deng, B.; Hu, P.; Fan, C.H.; Li, J.; Huang, Q. Preparation of polymer decorated graphene oxide by γ-ray induced graft polymerization. Nanoscale 2012, 4, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.T.; Pham, T.T.; Choi, J.Y.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development of a graphene oxide nanocarrier for dual-drug chemo-phototherapy to overcome drug resistance in cancer. ACS Appl. Mater. Interfaces 2015, 7, 28647–28655. [Google Scholar] [CrossRef] [PubMed]
- Nia, A.S.; Binder, W.H. Graphene as initiator/catalyst in polymerization chemistry. Prog. Polym. Sci. 2017, 67, 48–76. [Google Scholar]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H.J. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.H.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Bitounis, D.; Ali-Boucetta, H.; Hong, B.H.; Min, D.-H.; Kostarelos, K. Prospects and challenges of graphene in biomedical applications. Adv. Mater. 2013, 25, 2258–2268. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Li, J.C.; Shen, Z.H.; Zhong, C.L.; Liu, J.Y.; Ma, H.X.; Zhu, J.; Zhang, H.G.; Braun, P.V. Interlayer lithium plating in Au nanoparticles pillared reduced graphene oxide for lithium metal anodes. Adv. Funct. Mater. 2018, 48, 1804133. [Google Scholar] [CrossRef]
- Liu, S.; Cao, S.T.; Guo, J.Y.; Luo, L.Q.; Zhou, Y.; Lin, C.L.; Shi, J.Y.; Fan, C.H.; Lv, M.; Wang, L.H. Graphene oxide-silver nanocomposites modulate biofilm formation and extracellular polymeric substance (EPS) production. Nanoscale 2018, 10, 19603–19611. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, P. The use of nanocrystals in biological detection. Nat. Biotechnol. 2004, 22, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Ichikawa, S.; Maeda, Y.; Haruta, M.; Kohyama, M. Electronic structures of Au supported on TiO2. Appl. Catal. A 2005, 291, 45–54. [Google Scholar] [CrossRef]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of nanoparticles in biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, I.; Akita, T.; Okumura, M.; Haruta, M. Hydrogen dissociation by gold cluster. Angew. Chem. Int. Ed. 2009, 48, 9515–9518. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.L.; Wang, H.W.; Wang, Y.Y.; Ding, K.G.; Liu, H.; Yuan, L.; Shi, X.J.; Wang, M.M.; Wang, Y.W.; Chen, H. Maintaining the pluripotency of mouse embryonic stem cells on gold nanoparticle layers with nanoscale but not microscale surface roughness. Nanoscale 2014, 6, 6959–6969. [Google Scholar] [CrossRef] [PubMed]
- Jasuja, K.; Berry, V. Implantation and growth of dendritic gold nanostructures on graphene derivatives: Electricalproperty tailoring and Raman enhancement. ACS Nano 2009, 3, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Chen, B.; Ji, N.; Chen, F.; Zhang, Y.; Zhang, Z. Nanocomposites of size-controlled gold nanoparticles and graphene oxide: Formation and applications in SERS and catalysis. Nanoscale 2010, 2, 2733–2738. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ma, L.; Su, M.; Liu, D.; Wang, Z. Preparation and application of thionin-bridged graphene-gold nanoparticle nanohybrids. J. Mater. Chem. B 2013, 1, 1432–1438. [Google Scholar] [CrossRef]
- Han, S.T.; Zhou, Y.; Wang, C.; He, L.; Zhang, W.; Roy, V.A.L. Layer-by-layer-assembled reduced graphene oxide/gold nanoparticle hybrid double-floating-gate structure for low-voltage flexible flash memory. Adv. Mater. 2013, 25, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Govindhan, M.; Amiri, M.; Chen, A. Au nanoparticle/graphene nanocomposite as a platform for the sensitive detection of NADH in human urine. Biosens. Bioelectron. 2015, 66, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, J.; Camarada, M.B.; Venkatraman, D.; Ju, H.X.; Dey, R.S.; Wen, Y.P. One-step coelectrodeposition-assisted layer-by-layer assembly of gold nanoparticles and reduced graphene oxide and its self-healing three-dimensional nanohybrid for an ultrasensitive DNA sensor. Nanoscale 2018, 10, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Akbarian, F.; Heydari-Soureshjani, E.; Rezaei, B. A novel aptasensor based on 3D-reduced graphene oxide modified gold nanoparticles for determination of arsenite. Biosens. Bioelectron. 2018, 122, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Gracio, J. Surface modification of graphene nanosheets with gold nanoparticles: Therole of oxygen moieties at graphene surface on gold nucleation and growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Ismaili, H.; Geng, D.; Sun, A.X.; Kantzas, T.T.; Workentin, M.S. Light-activated covalent formation of gold nanoparticle-graphene and gold nanoparticle-glass composites. Langmuir 2011, 27, 13261–13268. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.A.; Choi, B.C.; Lim, K.T.; Jeong, Y.T. A simple approach for immobilization of gold nanoparticles on graphene oxide sheets by covalent bonding. Appl. Surf. Sci. 2011, 257, 3350–3357. [Google Scholar] [CrossRef]
- Leea, J.U.; Leea, W.; Yoona, S.S.; Kimb, J.; Byun, J.H. Site-selective immobilization of gold nanoparticles on graphene sheets and its electrochemical properties. Appl. Surf. Sci. 2014, 315, 73–80. [Google Scholar] [CrossRef]
- Liu, G.; Qi, M.; Zhang, Y.; Cao, C.; Goldys, E.M. Nanocomposites of gold nanoparticles and graphene oxide towards an stable label-free electrochemical immunosensor for detection of cardiac marker troponin-I. Anal. Chim. Acta 2016, 909, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Peniche-Covas, C.; Chico, B.; Simpson, B.K.; Villalonga, R. Ferrocene branched chitosan for the construction of a reagentless amperometric hydrogen peroxide biosensor. Macromol. Biosci. 2007, 7, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.H.; Li, G.Y.; Liang, J.T.; Su, J.; Zhang, Y.; Chen, H.Z.; Huang, Y.; Sui, W.G.; Zhao, Y.X. Non-enzymatic electrochemical biosensor based on Pt NPs/RGO-Cs-Fc nano-hybrids for the detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2016, 82, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Wu, Y.; Lu, F.; Chen, Y.; Gao, W. An electrochemiluminescence biosensor for endonuclease EcoRI detection. Biosens. Bioelectron. 2017, 89, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, Y.J.; Dai, J.; Lang, M.D.; Huang, X.Y. Functionalization of graphene oxide towards thermo- sensitive nanocomposites via moderate in situ SET-LRP. J. Polym. Sci. Polym. Chem. 2011, 49, 4747–4755. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, J.Z.; Li, Y.J.; Hu, J.H.; Yang, D.; Huang, X.Y. Thermoresponsive graphene oxide- PNIPAM nanocomposites with controllable grafting polymer chains via moderate in situ SET-LRP. J. Polym. Sci. Polym. Chem. 2012, 50, 4451–4458. [Google Scholar] [CrossRef]
- Brust, M.; Fink, J.; Bethell, D.; Schiffrin, D.J.; Kiely, C. Synthesis and reactions of functionalised gold nanoparticles. J. Chem. Soc. Chem. Commun. 1995, 1655–1656. [Google Scholar] [CrossRef]
- Oh, E.; Susumu, K.; Makinen, A.J.; Deschamps, J.R.; Huston, A.L.; Medintz, I.L. Colloidal stability of gold nanoparticles coated with multithiol-poly(ethylene glycol) ligands: Importance of structural constraints of the sulfur anchoring group. J. Phys. Chem. C 2013, 117, 18947–18956. [Google Scholar] [CrossRef]
- Mei, B.C.; Oh, E.; Susumu, K.; Farrell, D.; Mountziaris, T.J.; Mattoussi, H. Effects of ligand coordination number and surface curvature on the stability of gold nanoparticles in aqueous solutionss. Langmuir 2009, 25, 10604–10611. [Google Scholar] [CrossRef] [PubMed]
- Zopes, D.; Stein, B.; Mathur, S.; Graf, C. Improved stability of “naked” gold nanoparticles enabled by insitucoating with mono and multivalent thiol PEG ligands. Langmuir 2013, 29, 11217–11226. [Google Scholar] [CrossRef] [PubMed]
- Zaluzhna, O.; Li, Y.; Allison, T.C.; Tong, Y.J. Inverse-micelle-encapsulated water-enabled bond breaking of dialkyl diselenide/disulfide: A critical step for synthesizing high-quality gold nanoparticles. J. Am. Chem. Soc. 2012, 134, 17991–17996. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, R.; Beuermann, S. Fullerenes decorated with poly(vinylidene fluoride). Macromolecules 2011, 44, 2597–2603. [Google Scholar] [CrossRef]
- Megiel, E. Surface modification using TEMPO and its derivatives. Adv. Colloid Inter. Sci. 2017, 250, 158–184. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, H.; Ali, G.A.M.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in waste water treatment. J. Nanostruct. Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Sangermano, M.; Rodriguez, D.; Gonzalez, M.C.; Laurenti, E.; Yagci, Y. Visible light induced cationic polymerization of epoxides by using multiwalled carbon nanotubes. Macromol. Rapid Commun. 2018, 39, 1800250. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lu, G.L.; Huang, X.Y.; Li, Y.; Cao, F.Q.; Chen, H.; Liu, W.B. Thermo-responsive graphene oxide/poly(ethyl ethylene phosphate) nanocomposite via ring opening polymerization. Nanomaterials 2019, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.W.; Kuang, M.; Wang, D.Y.; Kurth, D.G.; Mohwald, H. Colloidally stable amphibious nanocrystals derived from poly(2-(dimethylamino)ethyl methacrylate) capping. Angew. Chem. Int. Ed. 2005, 44, 1717–1720. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Pan, X.C.; Wang, Z.Y.; Zhang, J.; Matyjaszewski, K. Influence of spacers in tetherable initiators on surface-initiated atom transfer radical polymerization (SI-ATRP). Macromolecules 2016, 49, 9283–9286. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Advanced materials by atom transfer radical polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M.; Tsujii, Y.; Fukuda, T. Controlled grafting of a well-defined polymer on a porous glass filter by surface-initiated atom transfer radical polymerization. Polymer 2001, 42, 6811–6815. [Google Scholar] [CrossRef]
- Husseman, M.; Malmstroem, E.E.; McNamara, M.; Mate, M.; Mecerreyes, D.; Benoit, D.G.; Hedrick, J.L.; Mansky, P.; Huang, E.; Russell, T.P.; et al. Controlled synthesis of polymer brushes by “living” free radical polymerization techniques. Macromolecules 1999, 32, 1424–1431. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Single-layer graphene nanosheets with controlled grafting of polymer chains. J. Mater. Chem. 2010, 20, 1982–1992. [Google Scholar] [CrossRef]
- Xu, P.; Yu, H.; Li, X. In situ growth of noble metal nanoparticles on graphene oxide sheets and direct construction of functionalized porous-layered structure on gravimetric microsensors for chemical detection. Chem. Commun. 2012, 48, 10784–10786. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Gu, L.N.; Yang, D.; Hu, J.H.; Lu, G.L.; Huang, X.Y. Size-controllable gold nanoparticles stabilized by PDEAEMA-based double hydrophilic graft copolymer. Polymer 2009, 50, 3990–3996. [Google Scholar] [CrossRef]
- Kaim, A.; Szydłowska, J.; Piotrowski, P.; Megiel, E. One-pot synthesis of gold nanoparticles densely coated with nitroxide spins. Polyhedron 2012, 46, 119–123. [Google Scholar] [CrossRef]
- Gozdziewska, M.; Cichowicz, G.; Markowska, K.; Zawada, K.; Megiel, E. Nitroxide-coated silver nanoparticles: Synthesis, surface physicochemistry and antibacterial activity. RSC Adv. 2015, 5, 58403–58415. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, W.; Song, T.; Ye, M.; Zhang, H.; Feng, C.; Lu, G.; Huang, X. Graphene Oxide/Ferrocene-Containing Polymer/Gold Nanoparticle Triple Nanocomposite. Nanomaterials 2019, 9, 310. https://doi.org/10.3390/nano9020310
Qian W, Song T, Ye M, Zhang H, Feng C, Lu G, Huang X. Graphene Oxide/Ferrocene-Containing Polymer/Gold Nanoparticle Triple Nanocomposite. Nanomaterials. 2019; 9(2):310. https://doi.org/10.3390/nano9020310
Chicago/Turabian StyleQian, Wenhao, Tao Song, Mao Ye, Haiyan Zhang, Chun Feng, Guolin Lu, and Xiaoyu Huang. 2019. "Graphene Oxide/Ferrocene-Containing Polymer/Gold Nanoparticle Triple Nanocomposite" Nanomaterials 9, no. 2: 310. https://doi.org/10.3390/nano9020310