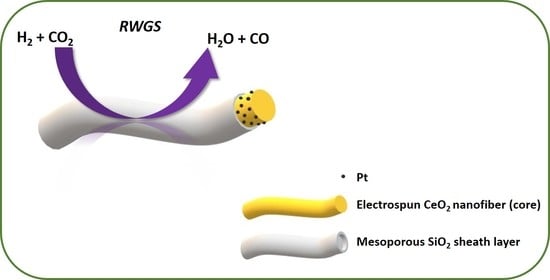
Core-Sheath Pt-CeO2/Mesoporous SiO2 Electrospun Nanofibers as Catalysts for the Reverse Water Gas Shift Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of CeO2 NFs
2.2.1. Preparation of Spinnable Solution
2.2.2. Axial Electrospinning of CeO2
2.3. Synthesis of CeO2NF@SiO2 Core-Sheath Structure
2.3.1. Preparation of Electrospun CeO2 NFs for Sol-Gel Synthesis
2.3.2. Sol-Gel Synthesis of CeO2 NF@SiO2 (CeSi)
2.4. Fabrication of Pt-CeO2 NF@SiO2
2.4.1. Wet Impregnation of Pt on Electrospun CeO2 NF
2.4.2. Preparation of Pre-Synthesized Pt NPs
2.4.3. Loading of Pre-Synthesized Pt on CeO2 NF by Solvothermal Method
2.4.4. Sol-Gel Synthesis of Pt-CeO2@SiO2
2.5. Characterization
2.6. Catalytic Activity Test
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dilamian, M.; Joghataei, M.; Ashrafi, Z.; Bohr, C.; Mathur, S.; Maleki, H. From 1D electrospun nanofibers to advanced multifunctional fibrous 3D aerogels. Appl. Mater. Today 2021, 22, 100964. [Google Scholar] [CrossRef]
- Garnett, E.; Mai, L.; Yang, P. Introduction: 1D nanomaterials/nanowires. Chem. Rev. 2019, 119, 8955–8957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, R.A.; Chang, C.-P.; Devan, R.S.; Liou, Y.; Ma, Y.-R. Impact of nanosize on supercapacitance: Study of 1D nanorods and 2D thin-films of nickel oxide. ACS Appl. Mater. Interfaces 2016, 8, 9872–9880. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, W.; Wang, B.; Zhang, W.; Zeng, X.; Zhang, C.; Qin, Y.; Sun, X.; Wu, T.; Liu, J. Regulating the spatial distribution of metal nanoparticles within metal-organic frameworks to enhance catalytic efficiency. Nat. Commun. 2017, 8, 14429. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C. One-Dimensional Nanostructures: Electrospinning Technique and Unique Nanofibers; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 3642364276. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Korotcenkov, G. Electrospun metal oxide nanofibers and their conductometric gas sensor application. Part 2: Gas sensors and their advantages and limitations. Nanomaterials 2021, 11, 1555. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, J.Y.; Yoon, S.K.; Choi, N.J. Fabrication of low-cost and high-performance coal fly ash nanofibrous membranes via electrospinning for the control of harmful substances. Fuel 2019, 237, 236–244. [Google Scholar] [CrossRef]
- Ge, J.C.; Wu, G.; Yoon, S.K.; Kim, M.S.; Choi, N.J. Study on the Preparation and Lipophilic Properties of Polyvinyl Alcohol (PVA) Nanofiber Membranes via Green Electrospinning. Nanomaterials 2021, 11, 2514. [Google Scholar] [CrossRef]
- Ehrmann, A. Non-toxic crosslinking of electrospun gelatin nanofibers for tissue engineering and biomedicine—A Review. Polymers 2021, 13, 1973. [Google Scholar] [CrossRef]
- Nageeb El-Helaly, S.; Abd-Elrasheed, E.; Salim, S.A.; Fahmy, R.H.; Salah, S.; EL-Ashmoony, M.M. Green Nanotechnology in the Formulation of a Novel Solid Dispersed Multilayered Core-Sheath Raloxifene-Loaded Nanofibrous Buccal Film; In Vitro and In Vivo Characterization. Pharmaceutics 2021, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, X.; Li, S.; Song, W.-L.; Yu, D.-G.; Annie Bligh, S.W. The Effect of Drug Heterogeneous Distributions within Core-Sheath Nanostructures on Its Sustained Release Profiles. Biomolecules 2021, 11, 1330. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A.; Bekheet, M.F.; Viter, R.; Miele, P.; Roualdes, S.; Bechelany, M. BN/GdxTi(1-x)O(4-x)/2 nanofibers for enhanced photocatalytic hydrogen production under visible light. Appl. Catal. B Environ. 2019, 251, 76–86. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Nada, A.A.; Bekheet, M.F.; Roualdes, S.; Riedel, W.; Iatsunskyi, I.; Coy, E.; Gurlo, A.; Bechelany, M. Coaxial nanofibers of nickel/gadolinium oxide/nickel oxide as highly effective electrocatalysts for hydrogen evolution reaction. J. Colloid Interface Sci. 2021, 587, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanloo, M.; Nada, A.A.; El-Maghrabi, H.H.; Bekheet, M.F.; Riedel, W.; Djamel, B.; Viter, R.; Roualdes, S.; Soliman, F.S.; Moustafa, Y.M.; et al. Superior efficiency of BN/Ce2O3/TiO2 nanofibers for photocatalytic hydrogen generation reactions. Appl. Surf. Sci. 2022, 594, 153438. [Google Scholar] [CrossRef]
- Wu, H.; Pan, W.; Lin, D.; Li, H. Electrospinning of ceramic nanofibers: Fabrication, assembly and applications. J. Adv. Ceram. 2012, 1, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of advanced electrospinning techniques: A critical review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Chang, K.; Zhang, H.; Cheng, M.; Lu, Q. Application of ceria in CO2 conversion catalysis. ACS Catal. 2019, 10, 613–631. [Google Scholar] [CrossRef]
- Kock, E.M.; Kogler, M.; Grunbacher, M.; Zhuo, C.; Thalinger, R.; Schmidmair, D.; Schlicker, L.; Gurlo, A.; Penner, S. Metastable Corundum-Type In2O3: Phase Stability, Reduction Properties, and Catalytic Characterization. J. Phys. Chem. C 2016, 120, 15272–15281. [Google Scholar] [CrossRef]
- Kock, E.M.; Kogler, M.; Zhuo, C.; Schlicker, L.; Bekheet, M.F.; Doran, A.; Gurlo, A.; Penner, S. Surface chemistry and stability of metastable corundum- type In2O3. Phys. Chem. Chem. Phys. 2017, 19, 19407–19419. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Ouyang, M.; Zhang, L.; Yang, M.; Wu, X.; Ran, R.; Weng, D.; Kang, F.; Si, Z. Single Atomic Pt on SrTiO3 Catalyst in Reverse Water Gas Shift Reactions. Catalysts 2021, 11, 738. [Google Scholar] [CrossRef]
- Chen, L.; Kovarik, L.; Szanyi, J. Temperature-Dependent Communication between Pt/Al2O3 Catalysts and Anatase TiO2 Dilutant: The Effects of Metal Migration and Carbon Transfer on the Reverse Water–Gas Shift Reaction. ACS Catal. 2021, 11, 12058–12067. [Google Scholar] [CrossRef]
- Chen, X.; Su, X.; Duan, H.; Liang, B.; Huang, Y.; Zhang, T. Catalytic performance of the Pt/TiO2 catalysts in reverse water gas shift reaction: Controlled product selectivity and a mechanism study. Catal. Today 2017, 281, 312–318. [Google Scholar] [CrossRef]
- Bekheet, M.F.; Grünbacher, M.; Schlicker, L.; Gili, A.; Doran, A.; Epping, J.D.; Gurlo, A.; Klötzer, B.; Penner, S. On the structural stability of crystalline ceria phases in undoped and acceptor-doped ceria materials under in situ reduction conditions. CrystEngComm 2019, 21, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Grünbacher, M.; Schlicker, L.; Bekheet, M.F.; Gurlo, A.; Klötzer, B.; Penner, S. H2 reduction of Gd- and Sm-doped ceria compared to pure CeO2 at high temperatures: Effect on structure, oxygen nonstoichiometry, hydrogen solubility and hydroxyl chemistry. Phys. Chem. Chem. Phys. 2018, 20, 22099–22113. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Lin, L.; Yao, S.; Liu, Z.; Zhang, F.; Li, N.; Vovchok, D.; Martinez-Arias, A.; Castañeda, R.; Lin, J.; Senanayake, S.D. In situ characterization of Cu/CeO2 nanocatalysts for CO2 hydrogenation: Morphological effects of nanostructured ceria on the catalytic activity. J. Phys. Chem. C 2018, 122, 12934–12943. [Google Scholar] [CrossRef]
- Yang, S.-C.; Pang, S.H.; Sulmonetti, T.P.; Su, W.-N.; Lee, J.-F.; Hwang, B.-J.; Jones, C.W. Synergy between ceria oxygen vacancies and Cu nanoparticles facilitates the catalytic conversion of CO2 to CO under mild conditions. ACS Catal. 2018, 8, 12056–12066. [Google Scholar] [CrossRef]
- Lyu, L.; Xie, Q.; Yang, Y.; Wang, R.; Cen, W.; Luo, S.; Yang, W.; Gao, Y.; Xiao, Q.; Zou, P. A novel CeO2 Hollow-Shell sensor constructed for high sensitivity of acetone gas detection. Appl. Surf. Sci. 2022, 571, 151337. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Liu, J.; Xu, C.; Jiang, G.; Duan, A. Design and Synthesis of 3D Ordered Macroporous CeO2-Supported Pt@ CeO2-δ Core–Shell Nanoparticle Materials for Enhanced Catalytic Activity of Soot Oxidation. Small 2013, 9, 3957–3963. [Google Scholar] [CrossRef]
- Bisht, A.; Gangwar, B.P.; Anupriya, T.; Sharma, S. Understanding the electrochemical differences of Pt doped and Pt supported over CeO2. J. Solid State Electrochem. 2014, 18, 197–206. [Google Scholar] [CrossRef]
- Bera, P.; Gayen, A.; Hegde, M.S.; Lalla, N.P.; Spadaro, L.; Frusteri, F.; Arena, F. Promoting effect of CeO2 in combustion synthesized Pt/CeO2 catalyst for CO oxidation. J. Phys. Chem. B 2003, 107, 6122–6130. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.; Chan, X.; Kim, T.J.; Kim, D.H. How Pt Interacts with CeO2 under the Reducing and Oxidizing Environments at Elevated Temperature: The Origin of Improved Thermal Stability of Pt/CeO2 Compared to CeO2. J. Phys. Chem. C 2016, 120, 25870–25879. [Google Scholar] [CrossRef]
- Goguet, A.; Meunier, F.C.; Tibiletti, D.; Breen, J.P.; Burch, R. Spectrokinetic Investigation of Reverse Water-Gas-Shift Reaction Intermediates over a Pt/CeO2 Catalyst. J. Phys. Chem. B 2004, 108, 20240–20246. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Su, X.; Liang, B.; Yang, X.; Ren, X.; Duan, H.; Huang, Y.; Zhang, T. Identification of relevant active sites and a mechanism study for reverse water gas shift reaction over Pt/CeO2 catalysts. J. Energy Chem. 2016, 25, 1051–1057. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, M.; Ma, P.; Zheng, Y.; Chen, J.; Li, H.; Zhang, X.; Zheng, K.; Kuang, Q.; Xie, Z.-X. Atomically dispersed Pt/CeO2 catalyst with superior CO selectivity in reverse water gas shift reaction. Appl. Catal. B Environ. 2021, 291, 120101. [Google Scholar] [CrossRef]
- Patel, A.C.; Li, S.; Wang, C.; Zhang, W.; Wei, Y. Electrospinning of porous silica nanofibers containing silver nanoparticles for catalytic applications. Chem. Mater. 2007, 19, 1231–1238. [Google Scholar] [CrossRef]
- Li, M.; Borsay, A.; Dakhchoune, M.; Zhao, K.; Luo, W.; Züttel, A. Thermal stability of size-selected copper nanoparticles: Effect of size, support and CO2 hydrogenation atmosphere. Appl. Surf. Sci. 2020, 510, 145439. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, P.; Cao, Z.; Campbell, C.T.; Xia, Y. The physical chemistry and materials science behind sinter-resistant catalysts. Chem. Soc. Rev. 2018, 47, 4314–4331. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Kerstien, J.; Schomäcker, R.; Thomas, A. Pd nanoparticles confined in mesoporous N-doped carbon silica supports: A synergistic effect between catalyst and support. Catal. Sci. Technol. 2020, 10, 1385–1394. [Google Scholar] [CrossRef] [Green Version]
- Joo, S.H.; Park, J.Y.; Tsung, C.-K.; Yamada, Y.; Yang, P.; Somorjai, G.A. Thermally stable Pt/mesoporous silica core–shell nanocatalysts for high-temperature reactions. Nat. Mater. 2009, 8, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.L.; He, W.; Gebbink, R.J.K.; de Jong, K.P. Palladium nanoparticles confined in thiol-functionalized ordered mesoporous silica for more stable Heck and Suzuki catalysts. Catal. Sci. Technol. 2015, 5, 1919–1928. [Google Scholar] [CrossRef]
- Su, J.; Xie, C.; Chen, C.; Yu, Y.; Kennedy, G.; Somorjai, G.A.; Yang, P. Insights into the mechanism of tandem alkene hydroformylation over a nanostructured catalyst with multiple interfaces. J. Am. Chem. Soc. 2016, 138, 11568–11574. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Murciano, L.; Chapman, R.S.L.; Narvaez-Dinamarca, A.; Mattia, D.; Jones, M.D. Effect of nanostructured ceria as support for the iron catalysed hydrogenation of CO2 into hydrocarbons. Phys. Chem. Chem. Phys. 2016, 18, 15496–15500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Q.; Shi, Z.; Wu, D. CO2 hydrogenation to methanol over a highly active Cu–Ni/CeO2–nanotube catalyst. Ind. Eng. Chem. Res. 2018, 57, 10148–10158. [Google Scholar] [CrossRef]
- Tang, H.; Sun, H.; Chen, D.; Jiao, X. Fabrication of Pt/CeO2 nanofibers for use in water–gas shift reaction. Mater. Lett. 2012, 77, 7–9. [Google Scholar] [CrossRef]
- Lu, P.; Qiao, B.; Lu, N.; Hyun, D.C.; Wang, J.; Kim, M.J.; Liu, J.; Xia, Y. Photochemical Deposition of Highly Dispersed Pt Nanoparticles on Porous CeO2 Nanofibers for the Water-Gas Shift Reaction. Adv. Funct. Mater. 2015, 25, 4153–4162. [Google Scholar] [CrossRef]
- Xie, C.; Chen, C.; Yu, Y.; Su, J.; Li, Y.; Somorjai, G.A.; Yang, P. Tandem catalysis for CO2 hydrogenation to C2–C4 hydrocarbons. Nano Lett. 2017, 17, 3798–3802. [Google Scholar] [CrossRef] [Green Version]
- Ryba, E. International Centre for Diffraction Data, Swarthmore, PA, $500.00. Powder Diffr. 1994, 9, 221–222. [Google Scholar] [CrossRef]
- File, P.D. JCPDS International Centre for Diffraction Data. ICDD, Powder Diffraction Journal, 13, Newtown Square, PA. 1998. Available online: https://www.icdd.com/powder-diffraction-journal-volume-13/#13-1 (accessed on 29 December 2022).
- Rodríguez-Carvajal, J. Recent developments of the program FULLPROF. Newsletter 2001, 26, 12–19. [Google Scholar]
- Osaki, T.; Narita, N.; Horiuchi, T.; Sugiyama, T.; Masuda, H.; Suzuki, K. Kinetics of reverse water gas shift (RWGS) reaction on metal disulfide catalysts. J. Mol. Catal. A Chem. 1997, 125, 63–71. [Google Scholar] [CrossRef]
- Dollase, W.A. Correction of intensities for preferred orientation in powder diffractometry: Application of the March model. J. Appl. Crystallogr. 1986, 19, 267–272. [Google Scholar] [CrossRef]
- San Andrés, E.; Del Prado, A.; Mártil, I.; González-Dıaz, G.; Bravo, D.; López, F.J.; Fernández, M.; Bohne, W.; Röhrich, J.; Selle, B. Bonding configuration and density of defects of SiOxHy thin films deposited by the electron cyclotron resonance plasma method. J. Appl. Phys. 2003, 94, 7462–7469. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Bai, H.; Huang, Y.; Liu, S.; Yen, S.; Tseng, Y. Synthesis of Neutral SiO2/TiO2 Hydrosol and Its Application as Antireflective Self-Cleaning Thin Film. Int. J. Photoenergy 2012, 2012, 620764. [Google Scholar] [CrossRef] [Green Version]
- Amin, M. Relationship Between the Pore Structure of Mesoporous Silica Supports and the Activity of Nickel Nanocatalysts in the CO2 Reforming of Methane. Catalysts 2020, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Yang, J.; Liang, J.; Guo, X.; Ding, W. In situ hydrothermal deposition as an efficient catalyst supporting method towards low-temperature graphitization of amorphous carbon. Carbon 2014, 77, 215–225. [Google Scholar] [CrossRef]
- Wang, G.D.; Kong, D.D.; Pan, Y.H.; Pan, H.B.; Zhu, J.F. Low energy Ar-ion bombardment effects on the CeO2 surface. Appl. Surf. Sci. 2012, 258, 2057–2061. [Google Scholar] [CrossRef]
- Holgado, J.P.; Alvarez, R.; Munuera, G. Study of CeO2 XPS spectra by factor analysis: Reduction of CeO2. Appl. Surf. Sci. 2000, 161, 301–315. [Google Scholar] [CrossRef]
- Holgado, J.P.; Munuera, G.; Espinós, J.P.; González-Elipe, A.R. XPS study of oxidation processes of CeOx defective layers. Appl. Surf. Sci. 2000, 158, 164–171. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D.; Pirone, R. CO and soot oxidation over Ce-Zr-Pr oxide catalysts. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhang, S.; Liu, B. Effect of oxygen vacancies on ceria catalyst for selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2020, 529, 147068. [Google Scholar] [CrossRef]
- Liu, P.; Niu, R.; Li, W.; Wang, S.; Li, J. Morphology effect of ceria on the ammonia synthesis activity of Ru/CeO2 catalysts. Catal. Lett. 2019, 149, 1007–1016. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corp. 1992, 40, 221. [Google Scholar]
- Sapkota, P.; Aprahamian, A.; Chan, K.Y.; Frentz, B.; Macon, K.T.; Ptasinska, S.; Robertson, D.; Manukyan, K. Irradiation-induced reactions at the CeO2/SiO2/Si interface. J. Chem. Phys. 2020, 152, 104704. [Google Scholar] [CrossRef] [PubMed]
- Paparazzo, E. On the number, binding energies, and mutual intensities of Ce3d peaks in the XPS analysis of cerium oxide systems: A response to Murugan et al., Superlatt. Microstruct. 85 (2015) 321. Superlattices Microstruct. 2017, 105, 216–220. [Google Scholar] [CrossRef]
- Romeo, M.; Bak, K.; El Fallah, J.; Le Normand, F.; Hilaire, L. XPS study of the reduction of cerium dioxide. Surf. Interface Anal. 1993, 20, 508–512. [Google Scholar] [CrossRef]
- Pfau, A.; Schierbaum, K.D. The electronic structure of stoichiometric and reduced CeO2 surfaces: An XPS, UPS and HREELS study. Surf. Sci. 1994, 321, 71–80. [Google Scholar] [CrossRef]
- Bera, P.; Anandan, C. Growth, Structural Characterization and Interfacial Reaction of Magnetron Sputtered CeO2 Thin Films on Different Substrates. Surf. Rev. Lett. 2014, 21, 1450054. [Google Scholar] [CrossRef]
- Yang, T.; Du, M.; Zhu, H.; Zhang, M.; Zou, M. Immobilization of Pt nanoparticles in carbon nanofibers: Bifunctional catalyst for hydrogen evolution and electrochemical sensor. Electrochim. Acta 2015, 167, 48–54. [Google Scholar] [CrossRef]
- Seo, P.W.; Choi, H.J.; Hong, S.I.; Hong, S.C. A study on the characteristics of CO oxidation at room temperature by metallic Pt. J. Hazard. Mater. 2010, 178, 917–925. [Google Scholar] [CrossRef]
- Mezni, A. Ternary hybrid Au@ Pt–TiO2 nanocomposites: Highly thermally stable photocatalyst with highly efficient visible-light photocatalytic activity. J. Mater. Res. Technol. 2020, 9, 15263–15272. [Google Scholar] [CrossRef]
- Bai, P.; Tian, F.; Wang, H.; Yang, T.; Bi, X.; Chai, Z.; Wang, X. Electrocatalytic Enhancement of 0D/1D/2D Multidimensional PtCo Alloy@ Cobalt Benzoate/Graphene Composite Catalyst for Alcohol Electro-Oxidation. Adv. Mater. Interfaces 2019, 6, 1900946. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Yin, H.; Hu, J.; Cheng, K.; Kang, J.; Zhang, Q.; Wang, Y. Tandem catalysis for hydrogenation of CO and CO2 to lower olefins with bifunctional catalysts composed of spinel oxide and SAPO-34. ACS Catal. 2020, 10, 8303–8314. [Google Scholar] [CrossRef]
- Wang, J.; Yin, G.; Shao, Y.; Zhang, S.; Wang, Z.; Gao, Y. Effect of carbon black support corrosion on the durability of Pt/C catalyst. J. Power Sources 2007, 171, 331–339. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mishra, A. Growth of PbBr2 microrods with unique structure and surface morphology. J. Mater. Sci. Mater. Electron. 2020, 31, 4672–4676. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, R.; Han, S.; Nandi, D.K.; Sawant, S.Y.; Kim, D.H.; Cheon, T.; Cho, M.H.; Harada, R.; Shigetomi, T.; Suzuki, K. Ultralow Loading (Single-Atom and Clusters) of the Pt Catalyst by Atomic Layer Deposition Using Dimethyl ((3, 4-η) N, N-dimethyl-3-butene-1-amine-N) Platinum (DDAP) on the High-Surface-Area Substrate for Hydrogen Evolution Reaction. Adv. Mater. Interfaces 2021, 8, 2001508. [Google Scholar] [CrossRef]
- Cui, J.; Ogabiela, E.; Hui, J.; Wang, Y.; Zhang, Y.; Tong, L.; Zhang, J.; Adeloju, S.; Zhang, X.; Wu, Y. Electrochemical Biosensor based on Pt/Au Alloy Nanowire Arrays for Phosphate Detection. J. Electrochem. Soc. 2015, 162, B62–B67. [Google Scholar] [CrossRef]
- Zhou, Y.; Perket, J.M.; Zhou, J. Growth of Pt nanoparticles on reducible CeO2 (111) thin films: Effect of nanostructures and redox properties of ceria. J. Phys. Chem. C 2010, 114, 11853–11860. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Chen, J.G.; Liu, P. CO2 hydrogenation on Pt, Pt/SiO2 and Pt/TiO2: Importance of synergy between Pt and oxide support. J. Catal. 2016, 343, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, D.; Kobayashi, H.; Kusada, K.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kawaguchi, S.; Kubota, Y.; Haneda, M.; Aspera, S.M. Boosting reverse water-gas shift reaction activity of Pt nanoparticles through light doping of W. J. Mater. Chem. A 2021, 9, 15613–15617. [Google Scholar] [CrossRef]
- Zhang, M.; Zijlstra, B.; Filot, I.A.W.; Li, F.; Wang, H.; Li, J.; Hensen, E.J.M. A theoretical study of the reverse water-gas shift reaction on Ni (111) and Ni (311) surfaces. Can. J. Chem. Eng. 2020, 98, 740–748. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.C.; Yao, Y.Y. Ceria in automotive exhaust catalysts: I. Oxygen storage. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- Chen, L.; Unocic, R.R.; Hoffman, A.S.; Hong, J.; Braga, A.H.; Bao, Z.; Bare, S.R.; Szanyi, J. Unlocking the Catalytic Potential of TiO2-Supported Pt Single Atoms for the Reverse Water–Gas Shift Reaction by Altering Their Chemical Environment. JACS Au 2021, 1, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Eom, H.; Kim, S.S. A study on the effect of CeO2 addition to a Pt/TiO2 catalyst on the reverse water gas shift reaction. Environ. Technol. 2021, 42, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Park, K.H.; Hong, S.C. A study of the selectivity of the reverse water–gas-shift reaction over Pt/TiO2 catalysts. Fuel Process. Technol. 2013, 108, 47–54. [Google Scholar] [CrossRef]
- Goguet, A.; Meunier, F.; Breen, J.P.; Burch, R.; Petch, M.I.; Ghenciu, A.F. Study of the origin of the deactivation of a Pt/CeO2 catalyst during reverse water gas shift (RWGS) reaction. J. Catal. 2004, 226, 382–392. [Google Scholar] [CrossRef]









| Sample | Material/Precursor | Preparation Method(s) | Heat Treatment/ Calcination Temperature (°C) | Desired Structure |
|---|---|---|---|---|
| Com-CeO2 | Commercial CeO2 | - | - | CeO2 powder |
| Ce(NO3)3·6H2O/PVP NF | Ce(NO3)3·6H2O/PVP | ES | No | Composite polymer/metal nitrate fibers |
| CeO2 NF | CeO2 | Calcination/ Ultrasonication | 600 | CeO2 fibers |
| IM-PtCe | [Pt(NH3)4](NO3)2-CeO2 | Wet-impregnation | 150 | Pt-CeO2 fibers |
| ST-PtCe | K2PtCl4-CeO2 | Solvothermal deposition | 150 | Pt-CeO2 fibers |
| IM-PtCeSi a | IM-PtCeO2-SiO2 | Sol-gel method | 360 | Core-sheath fibers (IM-Pt-CeO2 NF@SiO2) |
| ST-PtCeSi a | ST-PtCeO2-SiO2 | Sol-gel method | 360 | Core-sheath fibers (ST-Pt-CeO2 NF@SiO2) |
| Catalyst | Structure | Pt Loading | Temperature | GHSV | Ref. | ||
|---|---|---|---|---|---|---|---|
| %wt | °C | mL gcat−1 h−1 | % | % | |||
| Pt-CeO2 NF@SiO2 | Core-sheath NFs | 0.5 | 350 | 66,000 | 9 | ≈99 | This work |
| Pt-Al2O3 | Nanoparticles | 0.0125–0.25 | 300 | 80,000 | 9 | >99 | [23] |
| Pt-TiO2 | Nanoparticles | 0.025 and 2 | 250 | 80,000 | <2 | 100 | [85] |
| Pt-CeO2 | Nanorods | 0.3 | 350 | 72,000 | 8.5 | >98 | [37] |
| Pt-TiO2 | Nanoparticles | 0.5 | 400 | 6000 | 15 | ≈98 | [24] |
| Pt-CeO2 | Nanoparticles | 1 | 300 | 30,000 | 6.7 | NA | [36] |
| Pt/20%CeO2-TiO2 | Nanoparticles | 1 | 300 | 12,000 | 6.5 | NA | [86] |
| TiO2-supported Pt | Nanoparticles | 1 | 300 | 12,000 | 20 | NA | [87] |
| Pt-CeO2 | NA | 2 | 290 | 600,000 | 20 | NA | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nejadsalim, A.; Bashiri, N.; Godini, H.R.; Oliveira, R.L.; Tufail Shah, A.; Bekheet, M.F.; Thomas, A.; Schomäcker, R.; Gurlo, A.; Görke, O. Core-Sheath Pt-CeO2/Mesoporous SiO2 Electrospun Nanofibers as Catalysts for the Reverse Water Gas Shift Reaction. Nanomaterials 2023, 13, 485. https://doi.org/10.3390/nano13030485
Nejadsalim A, Bashiri N, Godini HR, Oliveira RL, Tufail Shah A, Bekheet MF, Thomas A, Schomäcker R, Gurlo A, Görke O. Core-Sheath Pt-CeO2/Mesoporous SiO2 Electrospun Nanofibers as Catalysts for the Reverse Water Gas Shift Reaction. Nanomaterials. 2023; 13(3):485. https://doi.org/10.3390/nano13030485
Chicago/Turabian StyleNejadsalim, Aidin, Najmeh Bashiri, Hamid Reza Godini, Rafael L. Oliveira, Asma Tufail Shah, Maged F. Bekheet, Arne Thomas, Reinhard Schomäcker, Aleksander Gurlo, and Oliver Görke. 2023. "Core-Sheath Pt-CeO2/Mesoporous SiO2 Electrospun Nanofibers as Catalysts for the Reverse Water Gas Shift Reaction" Nanomaterials 13, no. 3: 485. https://doi.org/10.3390/nano13030485