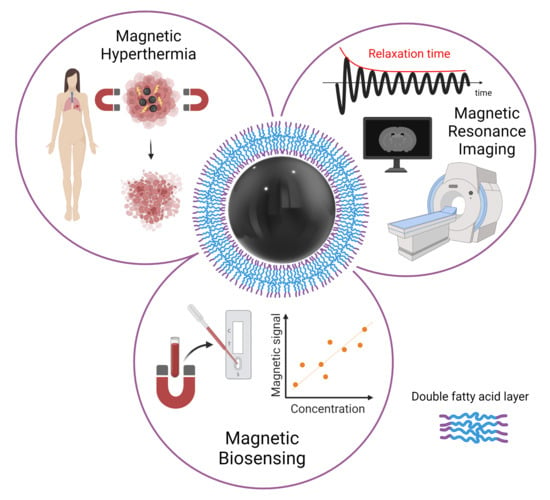
Double-Layer Fatty Acid Nanoparticles as a Multiplatform for Diagnostics and Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of the Magnetic Nanoparticles
2.3. Physicochemical and Magnetic Characterization of the Magnetic Nanoparticles
2.4. Magnetic Hyperthermia Measurements
2.5. Relaxometric Characterization
2.6. Biofunctionalization of the Magnetic Nanoparticles
2.7. Lateral Flow Assays
2.8. Scanning Magneto-Inductive Sensor Measurements
3. Results and Discussion
3.1. Physicochemical Characterization
3.2. Magnetic Properties
3.3. Magnetic Hyperthermia Measurements
3.4. Relaxometric Characterization
3.5. Biosensing Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hamilton, W. Cancer diagnostic delay in the COVID-19 era: What happens next? Lancet Oncol. 2020, 21, 1000–1002. [Google Scholar] [CrossRef]
- Blay, J.Y.; Boucher, S.; Le Vu, B.; Cropet, C.; Chabaud, S.; Perol, D.; Barranger, E.; Campone, M.; Conroy, T.; Coutant, C.; et al. Delayed care for patients with newly diagnosed cancer due to COVID-19 and estimated impact on cancer mortality in France. ESMO Open 2021, 6, 100134. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanomaterial-based devices for point-of-care diagnostic applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef] [PubMed]
- Chun, P. Colloidal Gold and Other Labels for Lateral Flow Immunoassays. In Lateral Flow Immunoassay; Humana Press: New York, NY, USA, 2009; pp. 1–19. [Google Scholar]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef] [Green Version]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. BioMetals 2016, 29, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.; Lim, M.; Goos, J.A.C.M.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchianò, V.; Salvador, M.; Moyano, A.; Gutiérrez, G.; Matos, M.; Yáñez-Vilar, S.; Piñeiro, Y.; Rivas, J.; Martínez-García, J.C.; Peddis, D.; et al. Electrodecoration and Characterization of Superparamagnetic Iron Oxide Nanoparticles with Bioactive Synergistic Nanocopper: Magnetic Hyperthermia-Induced Ionic Release for Anti-Biofilm Action. Antibiotics 2021, 10, 119. [Google Scholar] [CrossRef]
- Salvador, M.; Martínez-García, J.C.; Fernández-García, M.P.; Blanco-López, M.C.; Rivas, M. Biological and Medical Applications of Magnetic Nanoparticles. In Magnetic Measurement Techniques for Materials Characterization; Springer: Cham, Switzerland, 2021; pp. 771–804. [Google Scholar]
- Moyano, A.; Serrano-Pertierra, E.; Salvador, M.; Martínez-García, J.C.; Rivas, M.; Blanco-López, M.C. Magnetic lateral flow immunoassays. Diagnostics 2020, 10, 288. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Panferov, V.G.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Setting up the cut-off level of a sensitive barcode lateral flow assay with magnetic nanoparticles. Talanta 2017, 164, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hu, S.; Xiong, Y.; Wei, H.; Xu, H.; Duan, H.; Lai, W. Application and Development of Superparamagnetic Nanoparticles in Sample Pretreatment and Immunochromatographic Assay. TrAC-Trends Anal. Chem. 2019, 114, 151–170. [Google Scholar] [CrossRef]
- Lam, T.; Pouliot, P.; Avti, P.K.; Lesage, F.; Kakkar, A.K. Superparamagnetic iron oxide based nanoprobes for imaging and theranostics. Adv. Colloid Interface Sci. 2013, 199–200, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Stephen, Z.R.; Kievit, F.M.; Zhang, M. Magnetite nanoparticles for medical MR imaging. Mater. Today 2011, 14, 330–338. [Google Scholar] [CrossRef]
- Ortega, D.; Pankhurst, Q.A. Magnetic Hyperthermia; Nanoscience: Volume 1: Nanostructures through Chemistry; O’Brien, P., Ed.; Royal Society of Chemistry: Cambridge, UK, 2012; pp. 60–88. [Google Scholar]
- Moyano, A.; Salvador, M.; Martínez-García, J.C.; Socoliuc, V.; Vékás, L.; Peddis, D.; Alvarez, M.A.; Fernández, M.; Rivas, M.; Blanco-López, M.C. Magnetic immunochromatographic test for histamine detection in wine. Anal. Bioanal. Chem. 2019, 411, 6615–6624. [Google Scholar] [CrossRef]
- Moyano, A.; Serrano-Pertierra, E.; Duque, J.M.; Ramos, V.; Teruel-Barandiarán, E.; Fernández-Sánchez, M.T.; Salvador, M.; Martínez-García, J.C.; Sánchez, L.; García-Flórez, L.; et al. Magnetic Lateral Flow Immunoassay for Small Extracellular Vesicles Quantification: Application to Colorectal Cancer Biomarker Detection. Sensors 2021, 21, 3756. [Google Scholar] [CrossRef]
- Bica, D.; Vékás, L.; Avdeev, M.V.; Marinicǎ, O.; Socoliuc, V.; Bǎlǎsoiu, M.; Garamus, V.M. Sterically stabilized water based magnetic fluids: Synthesis, structure and properties. J. Magn. Magn. Mater. 2007, 311, 17–21. [Google Scholar] [CrossRef]
- Lago-Cachón, D.; Oliveira-Rodríguez, M.; Rivas, M.; Blanco-López, M.C.; Martínez-García, J.C.; Moyano, A.; Salvador, M.; García, J.A. Scanning Magneto-Inductive Sensor for Quantitative Assay of Prostate-Specific Antigen. In IEEE Magnetics Letters; IEEE: Piscataway, NJ, USA, 2017; Volume 8. [Google Scholar] [CrossRef]
- Muscas, G.; Singh, G.; Glomm, W.R.; Mathieu, R.; Kumar, P.A.; Concas, G.; Agostinelli, E.; Peddis, D. Tuning the size and shape of oxide nanoparticles by controlling oxygen content in the reaction environment: Morphological analysis by aspect maps. Chem. Mater. 2015, 27, 1982–1990. [Google Scholar] [CrossRef]
- Avdeev, M.V.; Mucha, B.; Lamszus, K.; Vékás, L.; Garamus, V.M.; Feoktystov, A.V.; Marinica, O.; Turcu, R.; Willumeit, R. Structure and in vitro biological testing of water-based ferrofluids stabilized by monocarboxylic acids. Langmuir 2010, 26, 8503–8509. [Google Scholar] [CrossRef]
- Borchert, H.; Shevchenko, E.V.; Robert, A.; Mekis, I.; Kornowski, A.; Grübel, G.; Weller, H. Determination of nanocrystal sizes: A comparison of TEM, SAXS, and XRD studies of highly monodisperse CoPt3 particles. Langmuir 2005, 21, 1931–1936. [Google Scholar] [CrossRef]
- Chantrell, R.W.; Popplewell, J.; Charles, S.W. Measurements of particle size distribution parameters in ferrofluids. IEEE Trans. Magn. 1978, 14, 975–977. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Wei, M.; Gu, H.; Xu, Q.; Zhu, W. Study of superparamagnetic nanoparticles as labels in the quantitative lateral flow immunoassay. Mater. Sci. Eng. C 2009, 29, 714–718. [Google Scholar] [CrossRef]
- Niculaes, D.; Lak, A.; Anyfantis, G.C.; Marras, S.; Laslett, O.; Avugadda, S.K.; Cassani, M.; Serantes, D.; Hovorka, O.; Chantrell, R.; et al. Asymmetric Assembling of Iron Oxide Nanocubes for Improving Magnetic Hyperthermia Performance. ACS Nano 2017, 11, 12121–12133. [Google Scholar] [CrossRef]
- Lan, Q.; Liu, C.; Yang, F.; Liu, S.; Xu, J.; Sun, D. Synthesis of bilayer oleic acid-coated Fe3O4 nanoparticles and their application in pH-responsive Pickering emulsions. J. Colloid Interface Sci. 2007, 310, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Lenin, R.; Joy, P.A. Role of Primary and Secondary Surfactant Layers on the Thermal Conductivity of Lauric Acid Coated Magnetite Nanofluids. J. Phys. Chem. C 2016, 120, 11640–11651. [Google Scholar] [CrossRef]
- Chen, M.J.; Shen, H.; Li, X.; Ruan, J.; Yuan, W.Q. Magnetic fluids’ stability improved by oleic acid bilayer-coated structure via one-pot synthesis. Chem. Pap. 2016, 70, 1642–1648. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Li, W.; Zaloga, J.; Ding, Y.; Liu, Y.; Janko, C.; Pischetsrieder, M.; Alexiou, C.; Boccaccini, A.R. Facile preparation of multifunctional superparamagnetic PHBV microspheres containing SPIONs for biomedical applications. Sci. Rep. 2016, 6, 23140. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Avdeev, M.V.; Bica, D.; Vekas, L.; Aksenov, V.L.; Feoktystov, A.V.; Rosta, L.; Garamus, V.M.; Willumeit, R. Structural aspects of stabilization of magnetic fluids by mono-carboxylic acids. In Proceedings of the Solid State Phenomena; Trans Tech Publications Ltd.: Baech, Switzerland, 2009; Volume 152, pp. 182–185. [Google Scholar]
- Bloemen, M.; Brullot, W.; Luong, T.T.; Geukens, N.; Gils, A.; Verbiest, T. Improved functionalization of oleic acid-coated iron oxide nanoparticles for biomedical applications. J. Nanoparticle Res. 2012, 14, 1100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zeng, D.; Liu, Z. The law of approach to saturation in ferromagnets originating from the magnetocrystalline anisotropy. J. Magn. Magn. Mater. 2010, 322, 2375–2380. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; Wiley: Weinheim, Germany, 2003; ISBN 9783527302741. [Google Scholar]
- Livesey, K.L.; Ruta, S.; Anderson, N.R.; Baldomir, D.; Chantrell, R.W.; Serantes, D. Beyond the blocking model to fit nanoparticle ZFC/FC magnetisation curves. Sci. Rep. 2018, 8, 11166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlfarth, E.P. Relations between Different Modes of Acquisition of the Remanent Magnetization of Ferromagnetic Particles. J. Appl. Phys. 2004, 29, 595. [Google Scholar] [CrossRef]
- Atkinson, W.J.; Brezovich, I.A.; Chakraborty, D.P. Usable Frequencies in Hyperthermia with Thermal Seeds. IEEE Trans. Biomed. Eng. 1984, 31, 70–75. [Google Scholar] [CrossRef]
- Lartigue, L.; Innocenti, C.; Kalaivani, T.; Awwad, A.; Sanchez Duque, M.D.M.; Guari, Y.; Larionova, J.; Gueírin, C.; Montero, J.L.G.; Barragan-Montero, V.; et al. Water-dispersible sugar-coated iron oxide nanoparticles. An evaluation of their relaxometric and magnetic hyperthermia properties. J. Am. Chem. Soc. 2011, 133, 10459–10472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasoialfari, A.; Filibian, M.; Sangregorio, C.; Carretta, P. In vivo biomedical applications of magnetic resonance and magnetic materials. Riv. Del. Nuovo Cim. 2013, 36, 211–271. [Google Scholar] [CrossRef]
- Haase, C.; Nowak, U. Role of dipole-dipole interactions for hyperthermia heating of magnetic nanoparticle ensembles. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 85, 045435. [Google Scholar] [CrossRef] [Green Version]
- Andreu, I.; Natividad, E.; Solozábal, L.; Roubeau, O. Nano-objects for addressing the control of nanoparticle arrangement and performance in magnetic hyperthermia. ACS Nano 2015, 9, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, U.; Buhl, E.M.; Baumann, M.; Schmitz-Rode, T.; Slabu, I. Agglomeration of magnetic nanoparticles and its effects on magnetic hyperthermia. Curr. Dir. Biomed. Eng. 2017, 3, 457–460. [Google Scholar] [CrossRef] [Green Version]
- Kruk, D.; Korpała, A.; Taheri, S.M.; Kozłowski, A.; Förster, S.; Rössler, E.A. 1H relaxation enhancement induced by nanoparticles in solutions: Influence of magnetic properties and diffusion. J. Chem. Phys. 2014, 140, 174504. [Google Scholar] [CrossRef] [PubMed]
- Basini, M.; Guerrini, A.; Cobianchi, M.; Orsini, F.; Bettega, D.; Avolio, M.; Innocenti, C.; Sangregorio, C.; Lascialfari, A.; Arosio, P. Tailoring the magnetic core of organic-coated iron oxides nanoparticles to influence their contrast efficiency for Magnetic Resonance Imaging. J. Alloys Compd. 2019, 770, 58–66. [Google Scholar] [CrossRef]
- Lago-Cachón, D.; Rivas, M.; Martínez-García, J.C.; Oliveira-Rodríguez, M.; Blanco-López, M.C.; García, J.A. High frequency lateral flow affinity assay using superparamagnetic nanoparticles. J. Magn. Magn. Mater. 2017, 423, 436–440. [Google Scholar] [CrossRef]
- Salvador, M.; Gallo-Cordova, Á.; Moyano, A.; Martínez-García, J.C.; Blanco-López, M.C.; Puerto Morales, M.; Rivas, M. Improved magnetic lateral flow assays with optimized nanotags for point-of-use inductive biosensing. Analyst 2020, 145, 5905–5914. [Google Scholar] [CrossRef]
- Sai, N.; Chen, Y.; Liu, N.; Yu, G.; Su, P.; Feng, Y.; Zhou, Z.; Liu, X.; Zhou, H.; Gao, Z.; et al. A sensitive immunoassay based on direct hapten coated format and biotin–streptavidin system for the detection of chloramphenicol. Talanta 2010, 82, 1113–1121. [Google Scholar] [CrossRef]
- Serebrennikova, K.V.; Samsonova, J.V.; Osipov, A.P. Enhancement of the Sensitivity of a Lateral Flow Immunoassay by Using the Biotin–Streptavidin System. Moscow Univ. Chem. Bull. 2018, 73, 131–134. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]







| Sample | ζ-Potential | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (nm) | σ | (nm) | ± | (nm) | σ | (nm) | PDI | (mV) | |
| OA@NP | 10.3 | 0.3 | 6.7 | 0.9 | 9.1 | 0.4 | 120 | 0.120 | –50 |
| LA@NP | 7.9 | 0.2 | 5.8 | 0.6 | 7.6 | 0.3 | 23 | 0.169 | −47 |
| MA@NP | 9.5 | 0.3 | 5.8 | 0.9 | 8.9 | 0.4 | 99 | 0.176 | −50 |
| Sample | χ′ | χ″ | |||||
|---|---|---|---|---|---|---|---|
| OA@NP | 71(1) | 79(1) | 0.024 | 0.37 | 2.2(2)·104 | 21.03 | 0.01 |
| LA@NP | 73(1) | 80(1) | 0.026 | 0.41 | 2.1(2)·104 | 15.88 | 0.03 |
| MA@NP | 67(1) | 74(1) | 0.016 | 0.25 | 1.9(2)·104 | 10.14 | 0.02 |
| Sample | (s−1·mmol−1·L) | (s−1·mmol−1·L) | (s−1·mmol−1·L) | (s−1·mmol−1·L) | (s−1·mmol−1·L) | (s−1·mmol−1·L) |
|---|---|---|---|---|---|---|
| OA@NP | 31.0 | 16.2 | 4.8 | 210.0 | 207.7 | 220.1 |
| LA@NP | 24.2 | 11.1 | 3.9 | 195.9 | 197.4 | 251.6 |
| MA@NP | 36.5 | 19.4 | 5.5 | 244.3 | 253.3 | 263.5 |
| Endorem® | 32.9 | 24.0 | 12.3 | 124.9 | 125.0 | 131.6 |
| Neutravidin Concentration | Peak 1 | Peak 2 | Peak 1 Volume Fraction (%) | Peak 2 Volume Fraction (%) | |
|---|---|---|---|---|---|
| (mg/mL) | |||||
| OA@NP | 0 | 45 | 158 | 34 | 66 |
| 0.75 | 59 | 208 | 25 | 75 | |
| 1 | 58 | 446 | 11 | 89 | |
| 2 | 107 | 553 | 9 | 91 | |
| LA@NP | 0 | 23 | 0 | 100 | 0 |
| 0.75 | 36 | 141 | 58 | 42 | |
| 1 | 38 | 149 | 45 | 55 | |
| 2 | 41 | 175 | 50 | 50 | |
| MA@NP | 0 | 46 | 141 | 44 | 56 |
| 0.75 | 105 | 442 | 26 | 74 | |
| 1 | 118 | 540 | 14 | 86 | |
| 2 | 53 | 212 | 15 | 85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador, M.; Marqués-Fernández, J.L.; Martínez-García, J.C.; Fiorani, D.; Arosio, P.; Avolio, M.; Brero, F.; Balanean, F.; Guerrini, A.; Sangregorio, C.; et al. Double-Layer Fatty Acid Nanoparticles as a Multiplatform for Diagnostics and Therapy. Nanomaterials 2022, 12, 205. https://doi.org/10.3390/nano12020205
Salvador M, Marqués-Fernández JL, Martínez-García JC, Fiorani D, Arosio P, Avolio M, Brero F, Balanean F, Guerrini A, Sangregorio C, et al. Double-Layer Fatty Acid Nanoparticles as a Multiplatform for Diagnostics and Therapy. Nanomaterials. 2022; 12(2):205. https://doi.org/10.3390/nano12020205
Chicago/Turabian StyleSalvador, María, José Luis Marqués-Fernández, José Carlos Martínez-García, Dino Fiorani, Paolo Arosio, Matteo Avolio, Francesca Brero, Florica Balanean, Andrea Guerrini, Claudio Sangregorio, and et al. 2022. "Double-Layer Fatty Acid Nanoparticles as a Multiplatform for Diagnostics and Therapy" Nanomaterials 12, no. 2: 205. https://doi.org/10.3390/nano12020205