Experimental Investigation and Molecular Dynamics Simulation on the Anti-Adhesion Behavior of Alkanethiols on Nickel Insert in Micro Injection Molding
Abstract
:1. Introduction
2. Experimental Trials
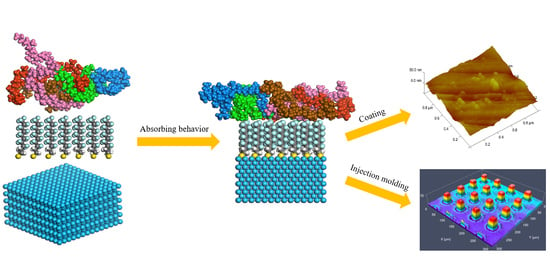
2.1. Anti-Adhesion Coating Preparation
2.2. Micro-Injection Molding Process
2.3. Effect of Anti-Adhesion Coating on the Replication Quality
3. Simulation Models and Methods
3.1. Materials and Model Construction
3.2. Force Field and Potential Function
3.3. Simulation Process
4. Results and Discussion
4.1. Evolution of Molecular Chains at the Interface
4.2. Anti-Adhesion Mechanism in P (2 × 2) Arrangement
4.3. Anti-Adhesion Mechanism in C (2 × 2) Arrangement
5. Conclusions
- (1)
- Experimental results showed that the self-assembled alkanethiol monolayers would not damage the micro-pillars and the surface roughness of the Ni mold insert. The sunken defects on the top surface of the micro-pillars almost disappeared. The alkanethiol coatings could effectively decrease the adhesion and promote the replication fidelity.
- (2)
- PP molecules were found to be adsorbed on the surface of alkanethiol monolayers in MD simulations, with relative concentration reaching the peak at the interface. After surface treatment, the adhesion work and the interfacial energy that mainly came from the vdW force were significantly reduced. The methyl groups in the PP layer and the hydrogen atom in alkanethiols were the main factors that affect the interfacial interaction.
- (3)
- When alkanethiol molecules were assembled in the P (2 × 2) arrangement, the adhesion at the PP–DT interface was the most contradictory. The PP–PFDT interface had the strongest electrostatic interaction energy due to the strong electronegativity of trifluoromethyl. When in the C (2 × 2) arrangement, PP molecules were not easy to adsorb on the PFDT surface due to the decrease in interaction energy.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Wang, H.; He, S.; Sa, T.; Cheng, X.; Xu, R. Design of micro-nano grooves incorporated into suspended GaN membrane for active integrated optics. AIP Adv. 2018, 8, 115118. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.A.; Leech, P.W. Optically variable watermark (OVW) microstructures for transparent substrates. Microelectron. Eng. 2006, 83, 2004–2008. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Z.; Wang, W.; Liu, W.; Xue, Q. Various curing conditions for controlling PTFE micro/nano-fiber texture of a bionic superhydrophobic coating surface. Mater. Chem. Phys. 2010, 119, 40–47. [Google Scholar] [CrossRef]
- Du, L.; Chang, H.; Song, M.; Liu, C. The effect of injection molding PMMA microfluidic chips thickness uniformity on the thermal bonding ratio of chips. Microsyst. Technol. 2012, 18, 815–822. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Zhou, M.; Weng, C. Self-assembled monolayers of alkanethiols on nickel insert: Characterization of friction and analysis on demolding quality in microinjection molding. Micromachines 2021, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Dhaduti, S.C.; Sarganachari, S.G.; Patil, A.Y.; Yunus Khan, T.M. Prediction of injection molding parameters for symmetric spur gear. J. Mol. Model. 2020, 26, 1–12. [Google Scholar] [CrossRef]
- Khan, M.; Khan, N.U.; Aziz, M.J.; Ullah, H.K.; Shahid, M.A. Improvement in power efficiency of injection molding machine by reduction in plasticization losses. Int. J. Manuf. Mater. Mech. Eng. 2016, 6, 62–76. [Google Scholar] [CrossRef]
- Jiang, K.; Yu, F.L.; Su, R.; Yang, J.H.; Zhou, T.N.; Gao, J.; Deng, H.; Wang, K.; Zhang, Q.; Chen, F.; et al. High speed injection molding of high density polyethylene—Effects of injection speed on structure and properties. Chin. J. Polym. Sci. 2011, 29, 456–464. [Google Scholar] [CrossRef]
- Kuo, C.C.; Chen, W.H.; Liu, X.Z.; Liao, Y.L.; Chen, W.J.; Huang, B.Y.; Tsai, R.L. Development of a low-cost wax injection mold with high cooling efficiency. Int. J. Adv. Manuf. Technol. 2017, 93, 2081–2088. [Google Scholar] [CrossRef]
- Medvedovski, E.; Peltsman, M. Low pressure injection moulding mass production technology of complex shape advanced ceramic components. Adv. Appl. Ceram. 2012, 111, 333–344. [Google Scholar] [CrossRef]
- Kazanskiy, N.L.; Stepanenko, I.S.; Khaimovich, A.I.; Kravchenko, S.V.; Byzov, E.V.; Moiseev, M.A. Injectional multilens molding parameters optimization. Comput. Opt. 2016, 40, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Weng, C.; Yang, J.; Yang, D.; Jiang, B. Molecular dynamics study on the deformation behaviors of nanostructures in the demolding process of micro-injection molding. Polymers 2019, 11, 470. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.L.; Ong, N.S.; Lam, Y.C. Mold surface roughness effects on cavity filling of polymer melt in micro injection molding. Int. J. Adv. Manuf. Technol. 2008, 37, 1105–1112. [Google Scholar] [CrossRef]
- Sasaki, T.; Koga, N.; Shirai, K.; Kobayashi, Y.; Toyoshima, A. Experimental study on ejection forces of injection molding. Precis. Eng. 2000, 24, 270–273. [Google Scholar] [CrossRef]
- Lee, N.; Choi, S.; Kang, S. Self-assembled monolayer as an antiadhesion layer on a nickel nanostamper in the nanoreplication process for optoelectronic applications. Appl. Phys. Lett. 2006, 88, 073101. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Cha, N.-G.; Lee, J.S.; Park, J.-G.; Carter, D.J.; Mead, J.L.; Barry, C.M.F. Effect of processing parameters, antistiction coatings, and polymer type when injection molding microfeatures. Polym. Eng. Sci. 2010, 50, 411–419. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, Y.; Park, J.; Im, S. Molecular simulation study on adhesions and deformations for polymethyl methacrylate (PMMA) resist in nanoimprint lithography. J. Mech. Sci. Technol. 2011, 25, 2311–2322. [Google Scholar] [CrossRef]
- Tsvetanova, M.; Oldenkotte, V.J.S.; Candelaria Bertolino, M.; Gao, Y.; Siekman, M.H.; Huskens, J.; Zandvliet, H.J.W.; Sotthewes, K. Nanoscale work function contrast induced by decanethiol self-assembled monolayers on Au(111). Langmuir 2020, 36, 12745–12754. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wu, K.Y.; Chao, Y.C.; Zan, H.W.; Meng, H.F.; Tao, Y.T. Concomitant tuning of metal work function and wetting property with mixed self-assembled monolayers. Org. Electron. 2011, 12, 148–153. [Google Scholar] [CrossRef]
- Pina-Estany, J.; García-Granada, A.A. Molecular dynamics simulation method applied to nanocavities replication via injection moulding. Int. Commun. Heat Mass Transf. 2017, 87, 1–5. [Google Scholar] [CrossRef]
- Zhou, M.; Xiong, X.; Drummer, D.; Jiang, B. Molecular dynamics simulation and experimental investigation of the geometrical morphology development of injection-molded nanopillars on polymethylmethacrylate surface. Comput. Mater. Sci. 2018, 149, 208–216. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, K.S.; Kim, K.W. Prediction of surface and adhesion energies of nanoimprint lithography materials and anti-sticking layers by molecular dynamics simulation. Appl. Surf. Sci. 2012, 258, 5438–5442. [Google Scholar] [CrossRef]
- Black, J.E.; Summers, A.Z.; Iacovella, C.R.; Cummings, P.T.; McCabe, C. Investigation of the impact of cross-polymerization on the structural and frictional properties of alkylsilane monolayers using molecular simulation. Nanomaterials 2019, 9, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Kang, N.; Zhou, J.; Li, X.; He, L.; Sui, H. Novel synthesis of choline-based amino acid ionic liquids and their applications for separating asphalt from carbonate rocks. Nanomaterials 2019, 9, 504. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Rao, Z. A molecular dynamics study on heat conduction of crosslinked epoxy resin based thermal interface materials for thermal management. Comput. Mater. Sci. 2020, 172, 109298. [Google Scholar] [CrossRef]
- Fattebert, J.L.; Richards, D.F.; Glosli, J.N. Dynamic load balancing algorithm for molecular dynamics based on Voronoi cells domain decompositions. Comput. Phys. Commun. 2012, 183, 2608–2615. [Google Scholar] [CrossRef]
- Gélébart, L.; Mondon-Cancel, R. Non-linear extension of FFT-based methods accelerated by conjugate gradients to evaluate the mechanical behavior of composite materials. Comput. Mater. Sci. 2013, 77, 430–439. [Google Scholar] [CrossRef]
- Wick, D.; Wick, T.; Hellmig, R.J.; Christ, H.J. Numerical simulations of crack propagation in screws with phase-field modeling. Comput. Mater. Sci. 2015, 109, 367–379. [Google Scholar] [CrossRef]
- Oed, W.; Starke, U.; Bothe, F.; Heinz, K. LEED structure analysis of p(2 × 2)S/Ni(100). Surf. Sci. 1990, 234, 72–78. [Google Scholar] [CrossRef]
- Dri, F.L.; Wu, X.; Moon, R.J.; Martini, A.; Zavattieri, P.D. Evaluation of reactive force fields for prediction of the thermo-mechanical properties of cellulose Iβ. Comput. Mater. Sci. 2015, 109, 330–340. [Google Scholar] [CrossRef] [Green Version]
- Fereidoon, A.; Aleaghaee, S.; Taraghi, I. Mechanical properties of hybrid graphene/TiO2 (rutile) nanocomposite: A molecular dynamics simulation. Comput. Mater. Sci. 2015, 102, 220–227. [Google Scholar] [CrossRef]
- Walker, C.C.; Genzer, J.; Santiso, E.E. Development of a fused-sphere SAFT-γ Mie force field for poly(vinyl alcohol) and poly(ethylene). J. Chem. Phys. 2019, 150, 034901. [Google Scholar] [CrossRef]
- Pechlaner, M.; Van Gunsteren, W.F. Algorithms to apply dihedral-angle constraints in molecular or stochastic dynamics simulations. J. Chem. Phys. 2020, 152, 024109. [Google Scholar] [CrossRef]
- Tan, C.; Jung, J.; Kobayashi, C.; Sugita, Y. A singularity-free torsion angle potential for coarse-grained molecular dynamics simulations. J. Chem. Phys. 2020, 153, 044110. [Google Scholar] [CrossRef]
- Yang, J.; Weng, C.; Lai, J.; Ding, T.; Wang, H. Molecular dynamics simulation on the influences of nanostructure shape, interfacial adhesion energy, and mold insert material on the demolding process of micro-injection molding. Polymers 2019, 11, 1573. [Google Scholar] [CrossRef] [Green Version]
- Schluttig, J.; Bachmann, M.J. Comparative molecular dynamics and Monte Carlo study of statistical properties for coarse-grained heteropolymers. J. Comput. Chem. 2008, 29, 2603–2612. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhai, Z.; Liu, J.; Weng, C. Molecular dynamics simulation on the adhesion mechanism at polymer-mold interface of microinjection molding. J. Appl. Polym. Sci. 2021, 138, 1–11. [Google Scholar] [CrossRef]











| Mold Temperature (°C) | Melt Temperature (°C) | Injection Velocity (mm3/s) | Packing Pressure (MPa) | Packing Time (s) | Cooling Time (s) |
|---|---|---|---|---|---|
| 100 | 250 | 18 | 140 | 10 | 60 |
| Material | Polymerization Degree | Number of Chains | Initial Density (g/cm3) | Temperature (K) | Box Size (nm) |
|---|---|---|---|---|---|
| PP | 30 | 5 | 0.9 | 298 | 3.24 × 3.24 × 2.24 |
| Interface Model | Einteraction (Kcal/mol) | vdW Energy (Kcal/mol) | Electrostatic Energy (Kcal/mol) | Adhesion Work (J/m2) | |
|---|---|---|---|---|---|
| Repulsive Energy | Dispersive Energy | ||||
| PP–Ni | −850.08 | 881.41 | −1731.49 | 0 | 0.56 |
| PP–DT | −83.64 | 79.33 | −169.76 | 6.79 | 0.06 |
| PP–DDT | −127.48 | 124.64 | −236.08 | −16.04 | 0.08 |
| PP–PFDT | −152.31 | 95.15 | −181.41 | −66.05 | 0.10 |
| Interface Model | Einteraction (Kcal/mol) | vdW Energy (Kcal/mol) | Electrostatic Energy (Kcal/mol) | Adhesion Work (J/m2) | |
|---|---|---|---|---|---|
| Repulsive Energy | Dispersive Energy | ||||
| PP–DT | −133.40 | 89.98 | −218.87 | −4.51 | 0.09 |
| PP–DDT | −186.25 | 173.14 | −342.78 | −16.61 | 0.12 |
| PP–PFDT | −116.40 | 50.18 | −103.70 | −62.88 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, C.; Chen, J.; Yang, J.; Zhou, M.; Jiang, B. Experimental Investigation and Molecular Dynamics Simulation on the Anti-Adhesion Behavior of Alkanethiols on Nickel Insert in Micro Injection Molding. Nanomaterials 2021, 11, 1834. https://doi.org/10.3390/nano11071834
Weng C, Chen J, Yang J, Zhou M, Jiang B. Experimental Investigation and Molecular Dynamics Simulation on the Anti-Adhesion Behavior of Alkanethiols on Nickel Insert in Micro Injection Molding. Nanomaterials. 2021; 11(7):1834. https://doi.org/10.3390/nano11071834
Chicago/Turabian StyleWeng, Can, Jiachen Chen, Jin Yang, Mingyong Zhou, and Bingyan Jiang. 2021. "Experimental Investigation and Molecular Dynamics Simulation on the Anti-Adhesion Behavior of Alkanethiols on Nickel Insert in Micro Injection Molding" Nanomaterials 11, no. 7: 1834. https://doi.org/10.3390/nano11071834