Improved Surface-Enhanced-Raman Scattering Sensitivity Using Si Nanowires/Silver Nanostructures by a Single Step Metal-Assisted Chemical Etching
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structure and Morphology
3.2. Optical Properties
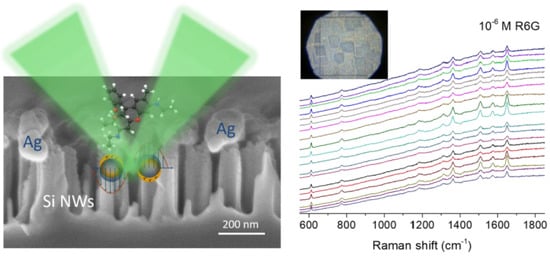
3.3. SERS Performance
3.3.1. A and B Series SERS Substrates
3.3.2. Homogeneity of SERS Spectra over the Surface of A- and B- Series Substrates
3.3.3. Enhancement Factors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Irudayaraj, J. Surface-enhanced Raman spectroscopy at single-molecule scale and its implications in biology. Philos. Trans. R. Soc. 2013, 368, 20120026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.-K.; Lee, K.S.; Shin, H.-H.; Koo, J.-J.; Yeon, G.J.; Kim, Z.H. Single-Molecule Surface-Enhanced Raman Scattering as a Probe of Single-Molecule Surface Reactions: Promises and Current Challenges. Acc. Chem. Res. 2019, 52, 3008–3017. [Google Scholar] [CrossRef]
- Almehmadi, L.M.; Curley, S.M.; Tokranova, N.A.; Tenenbaum, S.A.; Lednev, I.K. Surface Enhanced Raman Spectroscopy for Single Molecule Protein Detection. Sci. Rep. 2019, 9, 12356. [Google Scholar] [CrossRef] [Green Version]
- Zong, C.; Xu, M.; Xu, L.-J.; Wei, T.; Ma, X.; Zheng, X.-S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygenvacancies in materials. Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Segawa, H.; Fukuoka, T.; Itoh, T.; Imai, Y.; Iwata, Y.T.; Yamamuro, T.; Kuwayama, K.; Tsujikawa, K.; Kanamori, T.; Inoue, H. Rapid detection of hypnotics using surface-enhanced Raman scattering based on gold nanoparticle co-aggregation in a wet system. Analyst 2019, 144, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; He, L. Recent advance in SERS techniques for food safety and quality analysis: A brief review. Curr. Opin. Food Sci. 2019, 28, 82–87. [Google Scholar] [CrossRef]
- Blackie, E.J.; Le Ru, E.C.; Etchegoin, P.G. Single-Molecule Surface-Enhanced Raman Spectroscopy of Nonresonant Molecules. J. Am. Chem. Soc. 2009, 131, 14466–14472. [Google Scholar] [CrossRef] [PubMed]
- Galopin, E.; Barbillat, J.; Coffinier, Y.; Szunerits, S.; Patriarche, G.; Boukherroub, R. Silicon Nanowires Coated with Silver Nanostructures as Ultrasensitive Interfaces for Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2009, 1, 1396–1403. [Google Scholar] [CrossRef]
- Halouzka, V.; Jakubec, P.; Kvitek, L.; Likodimos, V.; Kontos, A.-G.; Papadopoulos, K.; Falaras, P.; Hrbac, J. Deposition of Nanostructured Ag Films on SiliconWafers by Electrochemical/Electrophoretic Deposition for Electrochemical and SERS Sensing. J. Electrochem. Soc. 2013, 160, B54–B59. [Google Scholar] [CrossRef]
- Halouzka, V.; Halouzkova, B.; Jirovskya, D.; Hemzal, D.; Ondra, P.; Siranidi, E.; Kontos, A.-G.; Polycarpos Falaras, P.; Hrbac, J. Copper nanowire coated carbon fibers as efficient substrates for detecting designer drugs using SERS. Talanta 2017, 165, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bjerneld, E.J.; Käll, M.; Börjesson, L. Spectroscopy of Single Hemoglobin Molecules by Surface Enhanced Raman Scattering. Phys. Rev. Lett. 1999, 83, 4357. [Google Scholar] [CrossRef] [Green Version]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Pan, R.; Cai, M.; Liu, W.; Chen, C.; Jiang, G.; Hu, X.; Zhang, H.; Zhong, M. Atto-Molar Raman detection on patterned superhydrophilic-superhydrophobic platform via localizable evaporation enrichment. Sens. Actuators B Chem. 2021, 326, 128826. [Google Scholar] [CrossRef]
- Bai, S.; Du, Y.; Wang, C.; Wu, J.; Sugioka, K. Reusable Surface-Enhanced Raman Spectroscopy Substrates Made of Silicon Nanowire Array Coated with Silver Nanoparticles Fabricated by Metal-Assisted Chemical Etching and Photonic Reduction. Nanomaterials 2019, 9, 1531. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Wu, Z.; Li, S.; Zhao, W.; Ma, C.; Wang, J.; Jiang, Z.; Zhong, Z.; Zheng, Y.; Yang, X. Large-Area Au-Nanoparticle-Functionalized Si Nanorod Arrays for Spatially Uniform Surface-Enhanced Raman Spectroscopy. ACS Nano 2017, 11, 1478–1487. [Google Scholar] [CrossRef]
- Huang, J.-A.; Zhao, Y.-Q.; Zhang, X.-J.; He, L.-F.; Wong, T.-L.; Chui, Y.-S.; Zhang, W.-J.; Lee, S.-T. Ordered Ag/Si Nanowires Array: Wide-Range Surface-Enhanced Raman Spectroscopy for Reproducible Biomolecule Detection. Nano Lett. 2013, 13, 5039–5045. [Google Scholar] [CrossRef]
- Lo Faro, M.J.; D’Andrea, C.; Leonardi, A.A.; Morganti, D.; Irrera, A.; Fazio, B. Fractal Silver Dendrites as 3D SERS Platform for Highly Sensitive Detection of Biomolecules in Hydration Conditions. Nanomaterials 2019, 9, 1630. [Google Scholar] [CrossRef] [Green Version]
- Leontis, Ι.; Botzakaki, M.A.; Georga, S.N.; Nassiopoulou, A.G. Study of Si Nanowires Produced by Metal-Assisted Chemical Etching as a Light-Trapping Material in n-type c-Si Solar Cells. ACS Omega 2018, 3, 10898–10906. [Google Scholar] [CrossRef]
- Leontis, Ι.; Botzakaki, M.A.; Georga, S.N.; Nassiopoulou, A.G. High capacitance density MIS capacitor using Si nanowires by MACE and ALD alumina dielectric. J. Appl. Phys. 2016, 119, 244508. [Google Scholar] [CrossRef]
- Sahoo, M.K.; Kale, P. Transfer of vertically aligned silicon nanowires array using sacrificial porous silicon layer. Thin Solid Films 2020, 698, 137866. [Google Scholar] [CrossRef]
- Green, M.A. Self-consistent optical parameters of intrinsic silicon at 300 K including temperature coefficients. Sol. Energy Mater. Sol. Cells 2008, 92, 1305–1310. [Google Scholar] [CrossRef]
- Bandarenka, H.V.; Girel, K.V.; Bondarenko, V.P.; Khodasevich, I.A.; Panarin, A.Y.; Terekhov, S.N. Formation Regularities of Plasmonic Silver Nanostructures on Porous Silicon for Effective Surface-Enhanced Raman Scattering. Nanoscale Res. Lett. 2016, 11, 262. [Google Scholar] [CrossRef] [Green Version]
- NʼGom, M.; Ringnalda, J.; Mansfield, J.F.; Agarwal, A.; Kotov, N.; Zaluzec, N.J.; Norris, T.B. Single Particle Plasmon Spectroscopy of Silver Nanowires and Gold Nanorods. Nano Lett. 2008, 8, 3200–3204. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Puebla, R.A. Effects of the Excitation Wavelength on the SERS Spectrum. J. Phys. Chem. Lett. 2012, 3, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Hayazawa, N.; Inouye, Y.; Kawata, S. DFT Vibrational Calculations of Rhodamine 6G Adsorbed on Silver: Analysis of Tip-Enhanced Raman Spectroscopy. J. Phys. Chem. B 2005, 109, 5012–5020. [Google Scholar] [CrossRef]
- Kudelski, A.; Pettinger, B. SERS on carbon chain segments: Monitoring locally surface chemistry. Chem. Phys. Lett. 2000, 321, 356–362. [Google Scholar] [CrossRef]
- Lu, Y.; Penzkofer, A. Absorption behaviour of methanolic rhodamine 6G solutions at high concentration. Chem. Phys. 1986, 107, 175–184. [Google Scholar] [CrossRef]
- Yang, S.; Dai, X.; Stogin, B.B.; Wong, T.-S. Ultrasensitive surface-enhanced Raman scattering detection in common fluids. Proc. Natl. Acad. Sci. USA 2016, 113, 268–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, F.; Gentile, F.; Mecarini, F.; Das, G.; Moretti, M.; Candeloro, P.; Coluccio, M.L.; Cojoc, G.; Accardo, A.; Liberale, C.; et al. Breaking the diffusion limit with super-hydrophobic delivery of molecules to plasmonic nanofocusing SERS structures. Nat. Photon 2011, 5, 682–687. [Google Scholar] [CrossRef]
- Shautsova, V.I.; Zhuravkov, V.A.; Korolik, O.V.; Novikau, A.G.; Shevchenko, G.P.; Gaiduk, P. Effect of Interparticle Field Enhancement in Self-Assembled Silver Aggregates on Surface-Enhanced Raman Scattering. Plasmonics 2014, 9, 993–999. [Google Scholar] [CrossRef]
- Mehigan, S.; Smyth, C.A.; McCabe, E.M. Bridging the Gap between SERS Enhancement and Reproducibility by Salt Aggregated Silver Nanoparticles. Nanomater. Nanotechnol. 2014, 5, 1–6. [Google Scholar] [CrossRef]






| SERS Substrates | First Method (A) | Second Method (B) | Etching Time (min, M) | Removal of Silver Residues | Re-Immersion Time (s, S) |
|---|---|---|---|---|---|
| A5M | YES | - | 5 | YES | - |
| A30M | YES | - | 30 | YES | - |
| A5M3S | YES | - | 5 | YES | 3 |
| A5M6S | YES | - | 5 | YES | 6 |
| A30M3S | YES | - | 30 | YES | 3 |
| A30M6S | YES | - | 30 | YES | 6 |
| A30M10S | YES | - | 30 | YES | 10 |
| B2M | - | YES | 2 | NO | - |
| B3.5M | - | YES | 3.5 | NO | - |
| B5M | - | YES | 5 | NO | - |
| SERS Sample | 10−6 M | 10−8 M | 10−10 M | 10−13 M |
|---|---|---|---|---|
| A5M3S | 2 × 105 | 2 × 107 | 7 × 108 | 1 × 1010 |
| A5M6S | 105 | 106 | - | - |
| B3.5M | 105 | 5 × 105 | - | - |
| SERS Sample | 10−6 M | 10−8 M | 10−10 M | 10−13 M |
|---|---|---|---|---|
| A5M3S | 2 × 105 | 2 × 107 | 5 × 108 | 6 × 109 |
| A5M6S | 105 | 6 × 105 | - | - |
| B3.5M | 105 | 3 × 105 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochylas, I.; Gardelis, S.; Likodimos, V.; Giannakopoulos, K.P.; Falaras, P.; Nassiopoulou, A.G. Improved Surface-Enhanced-Raman Scattering Sensitivity Using Si Nanowires/Silver Nanostructures by a Single Step Metal-Assisted Chemical Etching. Nanomaterials 2021, 11, 1760. https://doi.org/10.3390/nano11071760
Kochylas I, Gardelis S, Likodimos V, Giannakopoulos KP, Falaras P, Nassiopoulou AG. Improved Surface-Enhanced-Raman Scattering Sensitivity Using Si Nanowires/Silver Nanostructures by a Single Step Metal-Assisted Chemical Etching. Nanomaterials. 2021; 11(7):1760. https://doi.org/10.3390/nano11071760
Chicago/Turabian StyleKochylas, Ioannis, Spiros Gardelis, Vlassis Likodimos, Konstantinos P. Giannakopoulos, Polycarpos Falaras, and Androula G. Nassiopoulou. 2021. "Improved Surface-Enhanced-Raman Scattering Sensitivity Using Si Nanowires/Silver Nanostructures by a Single Step Metal-Assisted Chemical Etching" Nanomaterials 11, no. 7: 1760. https://doi.org/10.3390/nano11071760