Status Quo in Data Availability and Predictive Models of Nano-Mixture Toxicity
Abstract
:1. Introduction
2. Literature Collection
3. Current Available Predictive Models for Nano-Mixture Toxicity
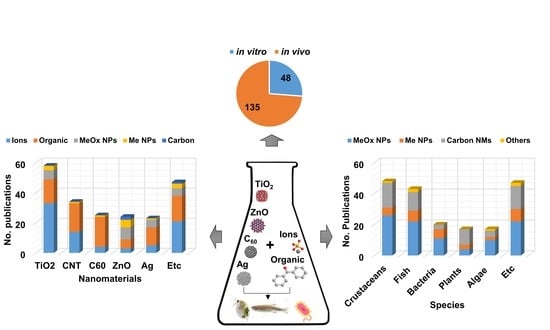
4. Current Available Data of Nano-Mixture Toxicity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Loewe, S.; Muischnek, H. Über Kombinationswirkungen I. Mitteilung: Hilfsmittel der Fragestellung. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1926, 114, 313–326. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Schaumann, G.E. Reliable predictive computational toxicology methods for mixture toxicity: Toward the development of innovative integrated models for environmental risk assessment. Rev. Environ. Sci. Biotechnol. 2013, 12, 235–256. [Google Scholar] [CrossRef]
- Bliss, C.I. The toxicity of poisons applied jointly 1. Ann. Appl. Biol. 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Gaudin, T.; Rotureau, P.; Fayet, G. Mixture Descriptors toward the Development of Quantitative Structure-Property Relationship Models for the Flash Points of Organic Mixtures. Ind. Eng. Chem. Res. 2015, 54, 6596–6604. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Ghosh, S.; Leszczynski, J. Single or mixture halogenated chemicals? Risk assessment and developmental toxicity prediction on zebrafish embryos based on weighted descriptors approach. Chemosphere 2018, 210, 588–596. [Google Scholar] [CrossRef]
- Qin, L.T.; Chen, Y.H.; Zhang, X.; Mo, L.Y.; Zeng, H.H.; Liang, Y.P. QSAR prediction of additive and non-additive mixture toxicities of antibiotics and pesticide. Chemosphere 2018, 198, 122–129. [Google Scholar] [CrossRef]
- Sobati, M.A.; Abooali, D.; Maghbooli, B.; Najafi, H. A new structure-based model for estimation of true critical volume of multi-component mixtures. Chemom. Intell. Lab. Syst. 2016, 155, 109–119. [Google Scholar] [CrossRef]
- Wang, T.; Tang, L.; Luan, F.; Cordeiro, M.N.D.S. Prediction of the toxicity of binary mixtures by qsar approach using the hypothetical descriptors. Int. J. Mol. Sci. 2018, 19, 3423. [Google Scholar] [CrossRef] [Green Version]
- Mikolajczyk, A.; Sizochenko, N.; Mulkiewicz, E.; Malankowska, A.; Rasulev, B.; Puzyn, T. A chemoinformatics approach for the characterization of hybrid nanomaterials: Safer and efficient design perspective. Nanoscale 2019, 11, 11808–11818. [Google Scholar] [CrossRef]
- Mikolajczyk, A.; Gajewicz, A.; Mulkiewicz, E.; Rasulev, B.; Marchelek, M.; Diak, M.; Hirano, S.; Zaleska-Medynska, A.; Puzyn, T. Nano-QSAR modeling for ecosafe design of heterogeneous TiO2-based nano-photocatalysts. Environ. Sci. Nano 2018, 5, 1150–1160. [Google Scholar] [CrossRef]
- Mikolajczyk, A.; Malankowska, A.; Nowaczyk, G.; Gajewicz, A.; Hirano, S.; Jurga, S.; Zaleska-Medynska, A.; Puzyn, T. Combined experimental and computational approach to developing efficient photocatalysts based on Au/Pd-TiO2 nanoparticles. Environ. Sci. Nano 2016, 3, 1425–1435. [Google Scholar] [CrossRef] [Green Version]
- Lopes, S.; Pinheiro, C.; Soares, A.M.V.M.; Loureiro, S. Joint toxicity prediction of nanoparticles and ionic counterparts: Simulating toxicity under a fate scenario. J. Hazard. Mater. 2016, 320, 1–9. [Google Scholar] [CrossRef]
- Azevedo, S.L.; Holz, T.; Rodrigues, J.; Monteiro, T.; Costa, F.M.; Soares, A.M.V.M.; Loureiro, S. A mixture toxicity approach to predict the toxicity of Ag decorated ZnO nanomaterials. Sci. Total Environ. 2017, 579, 337–344. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Wang, S.; Peijnenburg, W.J.G.M. Assessment and prediction of joint algal toxicity of binary mixtures of graphene and ionic liquids. Chemosphere 2017, 185, 681–689. [Google Scholar] [CrossRef]
- Martín-de-Lucía, I.; Gonçalves, S.F.; Leganés, F.; Fernández-Piñas, F.; Rosal, R.; Loureiro, S. Combined toxicity of graphite-diamond nanoparticles and thiabendazole to Daphnia magna. Sci. Total Environ. 2019, 688, 1145–1154. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Peijnenburg, W.J.G.M. Prediction of joint algal toxicity of nano-CeO2/nano-TiO2 and florfenicol: Independent action surpasses concentration addition. Chemosphere 2016, 156, 8–13. [Google Scholar] [CrossRef]
- Ye, N.; Wang, Z.; Wang, S.; Peijnenburg, W.J.G.M. Toxicity of mixtures of zinc oxide and graphene oxide nanoparticles to aquatic organisms of different trophic level: Particles outperform dissolved ions. Nanotoxicology 2018, 12, 423–438. [Google Scholar] [CrossRef]
- Altenburger, R.; Nendza, M.; Schuurmann, G. Mixture Toxicity and Its Modeling by Quantitative Structure–Activity Relationships. Environ. Toxicol. Chem. 2003, 22, 1900–1915. [Google Scholar] [CrossRef] [PubMed]
- Raies, A.B.; Bajic, V.B. In silico toxicology: Computational methods for the prediction of chemical toxicity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 147–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cedergreen, N. Quantifying synergy: A systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef] [PubMed]
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Wang, X.; Wang, Z.; Wei, Z.; Wang, L. Metal accumulation and antioxidant defenses in the freshwater fish Carassius auratus in response to single and combined exposure to cadmium and hydroxylated multi-walled carbon nanotubes. J. Hazard. Mater. 2014, 275, 89–98. [Google Scholar] [CrossRef]
- Lorenz, C.S.; Wicht, A.J.; Guluzada, L.; Crone, B.; Karst, U.; Lee, H.J.; Triebskorn, R.; Haderlein, S.B.; Huhn, C.; Köhler, H.R. Nano-sized zeolites as modulators of thiacloprid toxicity on Chironomus riparius. PeerJ 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, D.; Forthaus, B.E.; Wang, J. Quantifying the effect of nanoparticles on As(V) ecotoxicity exemplified by nano-Fe2O3 (magnetic) and nano-Al2O3. Environ. Toxicol. Chem. 2012, 31, 2870–2876. [Google Scholar] [CrossRef]
- Yu, Z.G.; Wang, W.X. Interaction of functionalized fullerenes and metal accumulation in Daphnia magna. Environ. Toxicol. Chem. 2014, 33, 1122–1128. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Xia, X.; Li, Y.; Zhou, Z.; Feng, C. Bioavailability of adsorbed phenanthrene by black carbon and multi-walled carbon nanotubes to Agrobacterium. Chemosphere 2010, 78, 1329–1336. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Wang, Z.; Ye, N.; Fang, H.; Wang, D. TiO2, SiO2 and ZrO2 nanoparticles synergistically provoke cellular oxidative damage in freshwater microalgae. Nanomaterials 2018, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Freitas, R.; Coppola, F.; De Marchi, L.; Codella, V.; Pretti, C.; Chiellini, F.; Morelli, A.; Polese, G.; Soares, A.M.V.M.; Figueira, E. The influence of Arsenic on the toxicity of carbon nanoparticles in bivalves. J. Hazard. Mater. 2018, 358, 484–493. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Shen, M.; Yin, Y.; Liu, J. Sunlight-driven reduction of silver ion to silver nanoparticle by organic matter mitigates the acute toxicity of silver to Daphnia magna. J. Environ. Sci. 2015, 35, 62–68. [Google Scholar] [CrossRef]
- Petersen, E.J.; Pinto, R.A.; Landrum, P.F.; Weber, W.J. Influence of carbon nanotubes on pyrene bioaccumulation from contaminated soils by earthworms. Environ. Sci. Technol. 2009, 43, 4181–4187. [Google Scholar] [CrossRef]
- Völker, C.; Gräf, T.; Schneider, I.; Oetken, M.; Oehlmann, J. Combined effects of silver nanoparticles and 17α-ethinylestradiol on the freshwater mudsnail Potamopyrgus antipodarum. Environ. Sci. Pollut. Res. 2014, 21, 10661–10670. [Google Scholar] [CrossRef]
- Wang, X.; Qu, R.; Liu, J.; Wei, Z.; Wang, L.; Yang, S.; Huang, Q.; Wang, Z. Effect of different carbon nanotubes on cadmium toxicity to Daphnia magna: The role of catalyst impurities and adsorption capacity. Environ. Pollut. 2016, 208, 732–738. [Google Scholar] [CrossRef]
- Iswarya, V.; Bhuvaneshwari, M.; Alex, S.A.; Iyer, S.; Chaudhuri, G.; Chandrasekaran, P.T.; Bhalerao, G.M.; Chakravarty, S.; Raichur, A.M.; Chandrasekaran, N.; et al. Combined toxicity of two crystalline phases (anatase and rutile) of Titania nanoparticles towards freshwater microalgae: Chlorella sp. Aquat. Toxicol. 2015, 161, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.B.; Wileman, S.J.; Boran, H.; Sutton, P. Association of Hg2+ with aqueous (C60)n aggregates facilitates increased bioavailability of Hg2+ in zebrafish (Danio rerio). Environ. Sci. Technol. 2013, 47, 9997–10004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cho, M.; Fortner, J.D.; Lee, J.; Huang, C.H.; Hughes, J.B.; Kim, J.H. Delineating oxidative processes of aqueous C60 preparations: Role of THF peroxide. Environ. Sci. Technol. 2009, 43, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Q.; Jiang, L.; Yu, Z.; Jiang, D.; Yin, D. Combined effects of titanium dioxide and humic acid on the bioaccumulation of cadmium in Zebrafish. Environ. Pollut. 2011, 159, 1151–1158. [Google Scholar] [CrossRef]
- Dalai, S.; Pakrashi, S.; Bhuvaneshwari, M.; Iswarya, V.; Chandrasekaran, N.; Mukherjee, A. Toxic effect of Cr(VI) in presence of n-TiO2 and n-Al2O3 particles towards freshwater microalgae. Aquat. Toxicol. 2014, 146, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.W.; Wang, Y.; Huang, B.; Wang, N.X.; Wei, Z.B.; Luo, J.; Miao, A.J.; Yang, L.Y. TiO2 nanoparticles act as a carrier of Cd bioaccumulation in the ciliate tetrahymena thermophila. Environ. Sci. Technol. 2014, 48, 7568–7575. [Google Scholar] [CrossRef]
- Sun, H.; Ruan, Y.; Zhu, H.; Zhang, Z.; Zhang, Y.; Yu, L. Enhanced bioaccumulation of pentachlorophenol in carp in the presence of multi-walled carbon nanotubes. Environ. Sci. Pollut. Res. 2014, 21, 2865–2875. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K.; Ahmad, I.; Rao, J.V.; Trindade, T.; Duarte, A.C.; Pereira, E. Modulation of glutathione and its dependent enzymes in gill cells of Anguilla anguilla exposed to silica coated iron oxide nanoparticles with or without mercury co-exposure under in vitro condition. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 162, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.W.; Miao, A.J.; Yang, L.Y. Cd2+ toxicity to a green alga Chlamydomonas reinhardtii as influenced by its adsorption on TiO2 engineered nanoparticles. PLoS ONE 2012, 7, e32300. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Romero, A.; Oron, M.; Cajaraville, M.P.; Soto, M.; Marigómez, I. Nanoparticle size and combined toxicity of TiO2 and DSLS (surfactant) contribute to lysosomal responses in digestive cells of mussels exposed to TiO2 nanoparticles. Nanotoxicology 2016, 10, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Kodali, V.K.; Roberts, J.R.; Shoeb, M.; Wolfarth, M.G.; Bishop, L.; Eye, T.; Barger, M.; Roach, K.A.; Friend, S.; Schwegler-Berry, D.; et al. Acute in vitro and in vivo toxicity of a commercial grade boron nitride nanotube mixture. Nanotoxicology 2017, 11, 1040–1058. [Google Scholar] [CrossRef]
- Chen, C.; Unrine, J.M.; Judy, J.D.; Lewis, R.W.; Guo, J.; McNear, D.H.; Tsyusko, O.V. Toxicogenomic Responses of the Model Legume Medicago truncatula to Aged Biosolids Containing a Mixture of Nanomaterials (TiO2, Ag, and ZnO) from a Pilot Wastewater Treatment Plant. Environ. Sci. Technol. 2015, 49, 8759–8768. [Google Scholar] [CrossRef]
- Martins, A.d.C.; Azevedo, L.F.; de Souza Rocha, C.C.; Carneiro, M.F.H.; Venancio, V.P.; de Almeida, M.R.; Antunes, L.M.G.; de Carvalho Hott, R.; Rodrigues, J.L.; Ogunjimi, A.T.; et al. Evaluation of distribution, redox parameters, and genotoxicity in Wistar rats co-exposed to silver and titanium dioxide nanoparticles. J. Toxicol. Environ. Health Part A Curr. Issues 2017, 80, 1156–1165. [Google Scholar] [CrossRef]
- Mohmood, I.; Ahmad, I.; Asim, M.; Costa, L.; Lopes, C.B.; Trindade, T.; Duarte, A.C.; Pereira, E. Interference of the co-exposure of mercury with silica-coated iron oxide nanoparticles can modulate genotoxicity induced by their individual exposures—A paradox depicted in fish under in vitro conditions. Environ. Sci. Pollut. Res. 2015, 22, 3687–3696. [Google Scholar] [CrossRef]
- Judy, J.D.; McNear, D.H.; Chen, C.; Lewis, R.W.; Tsyusko, O.V.; Bertsch, P.M.; Rao, W.; Stegemeier, J.; Lowry, G.V.; McGrath, S.P.; et al. Nanomaterials in Biosolids Inhibit Nodulation, Shift Microbial Community Composition, and Result in Increased Metal Uptake Relative to Bulk/Dissolved Metals. Environ. Sci. Technol. 2015, 49, 8751–8758. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Von der Kammer, F.; Hofmann, T.; Baalousha, M.; Ottofuelling, S.; Baun, A. Algal testing of titanium dioxide nanoparticles-Testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology 2010, 269, 190–197. [Google Scholar] [CrossRef]
- Alabresm, A.; Mirshahghassemi, S.; Chandler, G.T.; Decho, A.W.; Lead, J. Use of PVP-coated magnetite nanoparticles to ameliorate oil toxicity to an estuarine meiobenthic copepod and stimulate the growth of oil-degrading bacteria. Environ. Sci. Nano 2017, 4, 1859–1865. [Google Scholar] [CrossRef]
- Ginzburg, A.L.; Truong, L.; Tanguay, R.L.; Hutchison, J.E. Synergistic Toxicity Produced by Mixtures of Biocompatible Gold Nanoparticles and Widely Used Surfactants. ACS Nano 2018, 12, 5312–5322. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Duan, J.; Li, Y.; Yu, Y.; Jin, M.; Li, C.; Wang, Y.; Sun, Z. Combined toxicity of amorphous silica nanoparticles and methylmercury to human lung epithelial cells. Ecotoxicol. Environ. Saf. 2015, 112, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Waalewijn-Kool, P.L.; Zantkuijl, I.; Jurkschat, K.; Svendsen, C.; Soares, A.M.V.M.; Loureiro, S.; van Gestel, C.A.M. CeO2 nanoparticles induce no changes in phenanthrene toxicity to the soil organisms Porcellionides pruinosus and Folsomia candida. Ecotoxicol. Environ. Saf. 2015, 113, 201–206. [Google Scholar] [CrossRef]
- Santaella, C.; Allainmat, B.; Simonet, F.; Chanéac, C.; Labille, J.; Auffan, M.; Rose, J.; Achouak, W. Aged TiO2-based nanocomposite used in sunscreens produces singlet oxygen under long-wave UV and sensitizes escherichia coli to cadmium. Environ. Sci. Technol. 2014, 48, 5245–5253. [Google Scholar] [CrossRef]
- Henry, T.B.; Menn, F.M.; Fleming, J.T.; Wilgus, J.; Compton, R.N.; Sayler, G.S. Attributing effects of aqueous C60 nano-aggregates to tetrahydrofuran decomposition products in larval zebrafish by assessment of gene expression. Environ. Health Perspect. 2007, 115, 1059–1065. [Google Scholar] [CrossRef] [Green Version]
- Wilke, C.M.; Tong, T.; Gaillard, J.F.; Gray, K.A. Attenuation of Microbial Stress Due to Nano-Ag and Nano-TiO2 Interactions under Dark Conditions. Environ. Sci. Technol. 2016, 50, 11302–11310. [Google Scholar] [CrossRef]
- Huang, B.; Wei, Z.B.; Yang, L.Y.; Pan, K.; Miao, A.J. Combined Toxicity of Silver Nanoparticles with Hematite or Plastic Nanoparticles toward Two Freshwater Algae. Environ. Sci. Technol. 2019, 53, 3871–3879. [Google Scholar] [CrossRef]
- Sanchís, J.; Olmos, M.; Vincent, P.; Farré, M.; Barceló, D. New Insights on the Influence of Organic Co-Contaminants on the Aquatic Toxicology of Carbon Nanomaterials. Environ. Sci. Technol. 2016, 50, 961–969. [Google Scholar] [CrossRef]
- Park, H.G.; Yeo, M.K. Comparison of gene expression patterns from zebrafish embryos between pure silver nanomaterial and mixed silver nanomaterial containing cells of Hydra magnipapillata. Mol. Cell. Toxicol. 2015, 11, 307–314. [Google Scholar] [CrossRef]
- Hu, C.W.; Zhang, L.J.; Wang, W.L.; Cui, Y.B.; Li, M. Evaluation of the combined toxicity of multi-walled carbon nanotubes and sodium pentachlorophenate on the earthworm Eisenia fetida using avoidance bioassay and comet assay. Soil Biol. Biochem. 2014, 70, 123–130. [Google Scholar] [CrossRef]
- Nigro, M.; Bernardeschi, M.; Costagliola, D.; Della Torre, C.; Frenzilli, G.; Guidi, P.; Lucchesi, P.; Mottola, F.; Santonastaso, M.; Scarcelli, V.; et al. n-TiO2 and CdCl2 co-exposure to titanium dioxide nanoparticles and cadmium: Genomic, DNA and chromosomal damage evaluation in the marine fish European sea bass (Dicentrarchus labrax). Aquat. Toxicol. 2015, 168, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Moreno, D.; Valdehita, A.; Conde, E.; Rucandio, I.; Navas, J.M.; Fernández-Cruz, M.L. Acute toxic effects caused by the co-exposure of nanoparticles of ZnO and Cu in rainbow trout. Sci. Total Environ. 2019, 687, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, C.; Wang, W.; Ji, F.; Cui, Y.; Li, M. Acute toxicity of multi-walled carbon nanotubes, sodium pentachlorophenate, and their complex on earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2014, 103, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Soler de la Vega, A.C.; Molins-Delgado, D.; Barceló, D.; Díaz-Cruz, M.S. Nanosized titanium dioxide UV filter increases mixture toxicity when combined with parabens. Ecotoxicol. Environ. Saf. 2019, 184, 109565. [Google Scholar] [CrossRef]
- Naqvi, S.Z.H.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef] [Green Version]
- Zindler, F.; Glomstad, B.; Altin, D.; Liu, J.; Jenssen, B.M.; Booth, A.M. Phenanthrene bioavailability and toxicity to Daphnia magna in the presence of carbon nanotubes with different physicochemical properties. Environ. Sci. Technol. 2016, 50, 12446–12454. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.Y.; Xu, B.; Yu, C.P.; Zhang, H.W. Combined toxicity of ferroferric oxide nanoparticles and arsenic to the ciliated protozoa Tetrahymena Pyriformis. Aquat. Toxicol. 2013, 134–135, 66–73. [Google Scholar] [CrossRef]
- Zhai, Y.; Hunting, E.R.; Wouterse, M.; Peijnenburg, W.J.G.M.; Vijver, M.G. Importance of exposure dynamics of metal-based nano-ZnO, -Cu and -Pb governing the metabolic potential of soil bacterial communities. Ecotoxicol. Environ. Saf. 2017, 145, 349–358. [Google Scholar] [CrossRef]
- Svartz, G.; Papa, M.; Gosatti, M.; Jordán, M.; Soldati, A.; Samter, P.; Guraya, M.M.; Pérez Coll, C.; Perez Catán, S. Monitoring the ecotoxicity of γ-Al2O3 and Ni/γ-Al2O3 nanomaterials by means of a battery of bioassays. Ecotoxicol. Environ. Saf. 2017, 144, 200–207. [Google Scholar] [CrossRef]
- Rossi, S.C.; Mela, M.; Boschen, S.L.; da Cunha, C.; Neto, F.F.; Ribeiro, C.A.O.; Neves, A.P.P.; Silva de Assis, H.C. Modulatory effect of nano TiO2 on Pb in Hoplias malabaricus trophically exposed. Environ. Toxicol. Pharmacol. 2014, 38, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, L.; Yao, C.; Ding, L.; Li, C.; Fang, J.; Sui, K.; Liu, Y.; Wu, M. A combined toxicity study of zinc oxide nanoparticles and vitamin C in food additives. Nanoscale 2014, 6, 15333–15342. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Klaine, S.J.; Lin, S.; Ke, P.C.; Kim, S.D. Acute toxicity of a mixture of copper and single-walled carbon nanotubes to Daphnia magna. Environ. Toxicol. Chem. 2010, 29, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Peijnenburg, W.J.G.M.; Vijver, M.G. TiO2 nanoparticles reduce the effects of ZnO nanoparticles and Zn ions on zebrafish embryos (Danio rerio). NanoImpact 2016, 2, 45–53. [Google Scholar] [CrossRef]
- Cao, Y.; Roursgaard, M.; Kermanizadeh, A.; Loft, S.; Møller, P. Synergistic effects of zinc oxide nanoparticles and fatty acids on toxicity to caco-2 cells. Int. J. Toxicol. 2015, 34, 67–76. [Google Scholar] [CrossRef]
- Tsugita, M.; Morimoto, N.; Nakayama, M. SiO2 and TiO2 nanoparticles synergistically trigger macrophage inflammatory responses. Part. Fibre Toxicol. 2017, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pavagadhi, S.; Sathishkumar, M.; Balasubramanian, R. Uptake of Ag and TiO2 nanoparticles by zebrafish embryos in the presence of other contaminants in the aquatic environment. Water Res. 2014, 55, 280–291. [Google Scholar] [CrossRef]
- Fan, W.; Peng, R.; Li, X.; Ren, J.; Liu, T.; Wang, X. Effect of titanium dioxide nanoparticles on copper toxicity to Daphnia magna in water: Role of organic matter. Water Res. 2016, 105, 129–137. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Chen, J.; Tian, Y.; Shi, J.; Li, D.; Guo, C.; Ma, Q. Carboxylated multi-walled carbon nanotubes aggravated biochemical and subcellular damages in leaves of broad bean (Vicia faba L.) seedlings under combined stress of lead and cadmium. J. Hazard. Mater. 2014, 274, 404–412. [Google Scholar] [CrossRef]
- Jośko, I.; Oleszczuk, P.; Pranagal, J.; Lehmann, J.; Xing, B.; Cornelissen, G. Effect of biochars, activated carbon and multiwalled carbon nanotubes on phytotoxicity of sediment contaminated by inorganic and organic pollutants. Ecol. Eng. 2013, 60, 50–59. [Google Scholar] [CrossRef]
- Park, C.B.; Jung, J.W.; Yeom, D.H.; Jang, J.; Park, J.W.; Kim, Y.J. Interactive effects between components in binary mixtures of zinc sulfate and iron oxide nanoparticles on Daphnia magna. Mol. Cell. Toxicol. 2019, 15, 315–323. [Google Scholar] [CrossRef]
- Anjum, N.A.; Srikanth, K.; Mohmood, I.; Sayeed, I.; Trindade, T.; Duarte, A.C.; Pereira, E.; Ahmad, I. Brain glutathione redox system significance for the control of silica-coated magnetite nanoparticles with or without mercury co-exposures mediated oxidative stress in European eel (Anguilla anguilla L.). Environ. Sci. Pollut. Res. 2014, 21, 7746–7756. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, E.; Guilhermino, L. Are gold nanoparticles and microplastics mixtures more toxic to the marine microalgae Tetraselmis chuii than the substances individually? Ecotoxicol. Environ. Saf. 2019, 181, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Q.; Zhou, P.; Li, W.; Wang, J.; Huang, C.; Wang, X.; Lin, K.; Zhou, B. Bioconcentration and metabolism of BDE-209 in the presence of titanium dioxide nanoparticles and impact on the thyroid endocrine system and neuronal development in zebrafish larvae. Nanotoxicology 2014, 8, 196–207. [Google Scholar] [CrossRef]
- Renzi, M.; Blašković, A. Ecotoxicity of nano-metal oxides: A case study on Daphnia magna. Ecotoxicology 2019, 28, 878–889. [Google Scholar] [CrossRef]
- Sundukov, Y.N. First record of the ground beetle Trechoblemus postilenatus (Coleoptera, Carabidae) in Primorskii krai. Far East. Entomol. 2006, 165, 16. [Google Scholar] [CrossRef]
- Qiu, T.A.; Nguyen, T.H.T.; Hudson-Smith, N.V.; Clement, P.L.; Forester, D.C.; Frew, H.; Hang, M.N.; Murphy, C.J.; Hamers, R.J.; Feng, Z.V.; et al. Growth-Based Bacterial Viability Assay for Interference-Free and High-Throughput Toxicity Screening of Nanomaterials. Anal. Chem. 2017, 89, 2057–2064. [Google Scholar] [CrossRef]
- Polak, N.; Read, D.S.; Jurkschat, K.; Matzke, M.; Kelly, F.J.; Spurgeon, D.J.; Stürzenbaum, S.R. Metalloproteins and phytochelatin synthase may confer protection against zinc oxide nanoparticle induced toxicity in Caenorhabditis elegans. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 160, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Lee, B.T.; Kim, H.A.; Kim, K.W.; Kim, S.D.; Hwang, Y.S. Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of Daphnia magna. Chemosphere 2016, 143, 99–105. [Google Scholar] [CrossRef]
- Ogunsuyi, O.I.; Fadoju, O.M.; Akanni, O.O.; Alabi, O.A.; Alimba, C.G.; Cambier, S.; Eswara, S.; Gutleb, A.C.; Adaramoye, O.A.; Bakare, A.A. Genetic and systemic toxicity induced by silver and copper oxide nanoparticles, and their mixture in Clarias gariepinus (Burchell, 1822). Environ. Sci. Pollut. Res. 2019, 26, 27470–27481. [Google Scholar] [CrossRef]
- Fang, T.; Watson, J.L.; Goodman, J.; Dimkpa, C.O.; Martineau, N.; Das, S.; McLean, J.E.; Britt, D.W.; Anderson, A.J. Does doping with aluminum alter the effects of ZnO nanoparticles on the metabolism of soil pseudomonads? Microbiol. Res. 2013, 168, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Frenzilli, G.; Balbi, T.; Bernardeschi, M.; Ciacci, C.; Corsolini, S.; Della, C.; Fabbri, R.; Faleri, C.; Focardi, S.; et al. Interactive effects of n-TiO2 and 2,3,7,8-TCDD on the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 2014, 153, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Deville, S.; Baré, B.; Piella, J.; Tirez, K.; Hoet, P.; Monopoli, M.P.; Dawson, K.A.; Puntes, V.F.; Nelissen, I. Interaction of gold nanoparticles and nickel(II) sulfate affects dendritic cell maturation. Nanotoxicology 2016, 10, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Buonocore, F.; Frenzilli, G.; Corsolini, S.; Brunelli, A.; Guidi, P.; Kocan, A.; Mariottini, M.; Mottola, F.; Nigro, M.; et al. Influence of titanium dioxide nanoparticles on 2,3,7,8-tetrachlorodibenzo-p-dioxin bioconcentration and toxicity in the marine fish European sea bass (Dicentrarchus labrax). Environ. Pollut. 2015, 196, 185–193. [Google Scholar] [CrossRef]
- Della Torre, C.; Balbi, T.; Grassi, G.; Frenzilli, G.; Bernardeschi, M.; Smerilli, A.; Guidi, P.; Canesi, L.; Nigro, M.; Monaci, F.; et al. Titanium dioxide nanoparticles modulate the toxicological response to cadmium in the gills of Mytilus galloprovincialis. J. Hazard. Mater. 2015, 297, 92–100. [Google Scholar] [CrossRef]
- De La Rosa-García, S.C.; Martínez-Torres, P.; Gómez-Cornelio, S.; Corral-Aguado, M.A.; Quintana, P.; Gómez-Ortíz, N.M. Antifungal activity of ZnO and MgO nanomaterials and their mixtures against colletotrichum gloeosporioides strains from tropical fruit. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Brausch, K.A.; Anderson, T.A.; Smith, P.N.; Maul, J.D. Effects of functionalized fullerenes on bifenthrin and tribufos toxicity to Daphnia magna: Survival, reproduction, and growth rate. Environ. Toxicol. Chem. 2010, 29, 2600–2606. [Google Scholar] [CrossRef]
- Baun, A.; Sørensen, S.N.; Rasmussen, R.F.; Hartmann, N.B.; Koch, C.B. Toxicity and bioaccumulation of xenobiotic organic compounds in the presence of aqueous suspensions of aggregates of nano-C60. Aquat. Toxicol. 2008, 86, 379–387. [Google Scholar] [CrossRef]
- Balbi, T.; Smerilli, A.; Fabbri, R.; Ciacci, C.; Montagna, M.; Grasselli, E.; Brunelli, A.; Pojana, G.; Marcomini, A.; Gallo, G.; et al. Co-exposure to n-TiO2 and Cd2+ results in interactive effects on biomarker responses but not in increased toxicity in the marine bivalve M. galloprovincialis. Sci. Total Environ. 2014, 493, 355–364. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Adams, L.K.; Falkner, J.C.; Alvarez, P.J.J. Antibacterial activity of fullerene water suspensions: Effects of preparation method and particle size. Environ. Sci. Technol. 2006, 40, 4360–4366. [Google Scholar] [CrossRef]
- Baek, M.J.; Son, J.; Park, J.; Seol, Y.; Sung, B.; Kim, Y.J. Quantitative prediction of mixture toxicity of AgNO3 and ZnO nanoparticles on Daphnia magna. Sci. Technol. Adv. Mater. 2020, 21, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Worms, I.A.M.; Boltzman, J.; Garcia, M.; Slaveykova, V.I. Cell-wall-dependent effect of carboxyl-CdSe/ZnS quantum dots on lead and copper availability to green microalgae. Environ. Pollut. 2012, 167, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lin, B.; Hu, C.; Zhang, H.; Lin, Z.; Xi, Z. The combined toxicological effects of titanium dioxide nanoparticles and bisphenol A on zebrafish embryos. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdi, H.; De La Torre-Roche, R.; Hawthorne, J.; White, J.C. Impact of non-functionalized and amino-functionalized multiwall carbon nanotubes on pesticide uptake by lettuce (Lactuca sativa L.). Nanotoxicology 2014, 9, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Cai, Y.; Wang, W.; Cui, Y.; Li, M. Toxicological effects of multi-walled carbon nanotubes adsorbed with nonylphenol on earthworm Eisenia fetida. Environ. Sci. Process. Impacts 2013, 15, 2125–2130. [Google Scholar] [CrossRef]
- Fan, W.H.; Cui, M.M.; Shi, Z.W.; Tan, C.; Yang, X.P. Enhanced oxidative stress and physiological damage in Daphnia magna by copper in the presence of Nano-TiO2. J. Nanomater. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Cui, M.; Liu, H.; Wang, C.; Shi, Z.; Tan, C.; Yang, X. Nano-TiO2 enhances the toxicity of copper in natural water to Daphnia magna. Environ. Pollut. 2011, 159, 729–734. [Google Scholar] [CrossRef]
- Tian, S.Y.; Gao, Y.N.; Song, C.Z. Acute toxicities of penta-BDE in TiO2 nanoparticle suspensions to Daphnia magna. Adv. Mater. Res. 2014, 864–867, 261–265. [Google Scholar] [CrossRef]
- Tan, C.; Wang, W.X. Modification of metal bioaccumulation and toxicity in Daphnia magna by titanium dioxide nanoparticles. Environ. Pollut. 2014, 186, 36–42. [Google Scholar] [CrossRef]
- Seitz, F.; Bundschuh, M.; Dabrunz, A.; Bandow, N.; Schaumann, G.E.; Schulz, R. Titanium dioxide nanoparticles detoxify pirimicarb under UV irradiation at ambient intensities. Environ. Toxicol. Chem. 2012, 31, 518–523. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, Z.; Xia, J.; Wang, K.; Ling, X.; Yan, B. Potential toxicity in crucian carp following exposure to metallic nanoparticles of copper, chromium, and their mixtures: A comparative study. Polish J. Environ. Stud. 2017, 26, 2085–2094. [Google Scholar] [CrossRef]
- Rosenfeldt, R.R.; Seitz, F.; Schulz, R.; Bundschuh, M. Heavy metal uptake and toxicity in the presence of titanium dioxide nanoparticles: A factorial approach using Daphnia magna. Environ. Sci. Technol. 2014, 48, 6965–6972. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, R.R.; Seitz, F.; Senn, L.; Schilde, C.; Schulz, R.; Bundschuh, M. Nanosized titanium dioxide reduces copper toxicity-the role of organic material and the crystalline phase. Environ. Sci. Technol. 2015, 49, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Legros, S.; Von der Kammer, F.; Hofmann, T.; Baun, A. The potential of TiO2 nanoparticles as carriers for cadmium uptake in Lumbriculus variegatus and Daphnia magna. Aquat. Toxicol. 2012, 118–119, 1–8. [Google Scholar] [CrossRef]
- Wang, D.; Hu, J.; Irons, D.R.; Wang, J. Synergistic toxic effect of nano-TiO2 and As(V) on Ceriodaphnia dubia. Sci. Total Environ. 2011, 409, 1351–1356. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.; Hawthorne, J.; Musante, C.; Xing, B.; Newman, L.A.; Ma, X.; White, J.C. Impact of Ag nanoparticle exposure on p,p′-DDE bioaccumulation by cucurbita pepo (Zucchini) and glycine max (Soybean). Environ. Sci. Technol. 2013, 47, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Jung, J.W.; Baek, M.; Sung, B.; Park, J.W.J.W.; Seol, Y.; Yeom, D.H.; Park, J.W.J.W.; Kim, Y.J. Mixture toxicity of metal oxide nanoparticles and silver ions on Daphnia magna. J. Nanoparticle Res. 2019, 21, 21. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, H.; Zhang, Z.; Niu, Q.; Chen, Y.; Crittenden, J.C. Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere 2007, 67, 160–166. [Google Scholar] [CrossRef]
- Miao, W.; Zhu, B.; Xiao, X.; Li, Y.; Dirbaba, N.B.; Zhou, B.; Wu, H. Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat. Toxicol. 2015, 161, 117–126. [Google Scholar] [CrossRef]
- Tong, T.; Wilke, C.M.; Wu, J.; Binh, C.T.T.; Kelly, J.J.; Gaillard, J.F.; Gray, K.A. Combined Toxicity of Nano-ZnO and Nano-TiO2: From Single- to Multinanomaterial Systems. Environ. Sci. Technol. 2015, 49, 8113–8123. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, K.; Han, G.; Yu, M.; Chi, T.; Jing, S.; Li, Z.; Zhan, J.; Wu, M. Toxic effect of triphenyltin in the presence of nano zinc oxide to marine copepod Tigriopus japonicus. Environ. Pollut. 2018, 243, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, Y.; Song, C.; Zhu, X.; Xing, B. Titanium dioxide nanoparticles as carrier facilitate bioaccumulation of phenanthrene in marine bivalve, ark shell (Scapharca subcrenata). Environ. Pollut. 2014, 192, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Hwang, J.; Kim, J.; Jeong, Y.; Hwang, M.P.; Choi, J. Antibacterial activity and cytotoxicity of multi-walled carbon nanotubes decorated with silver nanoparticles. Int. J. Nanomed. 2014, 9, 4621–4629. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.Y.; Jia, F.; Chen, Y.X.; Gan, J. Influence of single-walled carbon nanotubes on microbial availability of phenanthrene in sediment. Ecotoxicology 2011, 20, 1277–1285. [Google Scholar] [CrossRef]
- Rocco, L.; Santonastaso, M.; Nigro, M.; Mottola, F.; Costagliola, D.; Bernardeschi, M.; Guidi, P.; Lucchesi, P.; Scarcelli, V.; Corsi, I.; et al. Genomic and chromosomal damage in the marine mussel Mytilus galloprovincialis: Effects of the combined exposure to titanium dioxide nanoparticles and cadmium chloride. Mar. Environ. Res. 2015, 111, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Lammel, T.; Wassmur, B.; Mackevica, A.; Chen, C.E.L.; Sturve, J. Mixture toxicity effects and uptake of titanium dioxide (TiO2) nanoparticles and 3,3″,4,4″-tetrachlorobiphenyl (PCB77) in juvenile brown trout following co-exposure via the diet. Aquat. Toxicol. 2019, 213, 105195. [Google Scholar] [CrossRef]
- Chen, F.; Wu, L.; Xiao, X.; Rong, L.; Li, M.; Zou, X. Mixture toxicity of zinc oxide nanoparticle and chemicals with different mode of action upon Vibrio fischeri. Environ. Sci. Eur. 2020, 32. [Google Scholar] [CrossRef]
- Vannuccini, M.L.; Grassi, G.; Leaver, M.J.; Corsi, I. Combination effects of nano-TiO2 and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on biotransformation gene expression in the liver of European sea bass Dicentrarchus labrax. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 176–177, 71–78. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Ferreira, J.L.R.; Lonné, M.N.; França, T.A.; Maximilla, N.R.; Lugokenski, T.H.; Costa, P.G.; Fillmann, G.; Antunes Soares, F.A.; de la Torre, F.R.; Monserrat, J.M. Co-exposure of the organic nanomaterial fullerene C60 with benzo[a]pyrene in Danio rerio (zebrafish) hepatocytes: Evidence of toxicological interactions. Aquat. Toxicol. 2014, 147, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahboob, S.; Al-Ghanim, K.A.; Al-Mulhim, N.M.A. Fish Exposure to Sub-Lethal Toxicity of Nano-Titanium Oxide and Changes in Muscular Antioxidant Enzymes and Protective Role of Vitamins C and E in Clarias gariepinus. Int. J. Agric. Biol. 2017, 19, 1505–1510. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, A. Comparative cytotoxicity of nanoparticles and ions to Escherichia coli in binary mixtures. J. Environ. Sci. 2017, 55, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, J.; Wu, C.; Wu, Q.; Li, J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 2005, 16, 1912–1917. [Google Scholar] [CrossRef]
- Kühnel, D.; Busch, W.; Meißner, T.; Springer, A.; Potthoff, A.; Richter, V.; Gelinsky, M.; Scholz, S.; Schirmer, K. Agglomeration of tungsten carbide nanoparticles in exposure medium does not prevent uptake and toxicity toward a rainbow trout gill cell line. Aquat. Toxicol. 2009, 93, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Edgington, A.J.; Klaine, S.J.; Cho, J.W.; Kim, S.D. Influence of multiwalled carbon nanotubes dispersed in natural organic matter on speciation and bioavailability of copper. Environ. Sci. Technol. 2009, 43, 8979–8984. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Shi, F.; Li, R.; Liu, L.; Liu, Y.; Liu, F. Interactive effects of cadmium and carbon nanotubes on the growth and metal accumulation in a halophyte Spartina alterniflora (Poaceae). Plant Growth Regul. 2013, 71, 171–179. [Google Scholar] [CrossRef]
- Cuahtecontzi-Delint, R.; Mendez-Rojas, M.A.; Bandala, E.R.; Quiroz, M.A.; Recillas, S.; Sanchez-Salas, J.L. Enhanced antibacterial activity of CeO2 nanoparticles by surfactants. Int. J. Chem. React. Eng. 2013, 11, 781–785. [Google Scholar] [CrossRef]
- Kelsey, J.W.; White, J.C. Effect of C60 fullerenes on the accumulation of weathered p,p′-DDE by plant and earthworm species under single and multispecies conditions. Environ. Toxicol. Chem. 2013, 32, 1117–1123. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, X.; Chen, L.; Lin, K.F.; Dong, Q.X.; Huang, C.J.; Fu, R.B.; Zhu, J. Toxicological effect of joint cadmium selenium quantum dots and copper ion exposure on zebrafish. Environ. Toxicol. Chem. 2012, 31, 2117–2123. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Henry, T.B.; Menn, F.M.; Compton, R.N.; Sayler, G. No bioavailability of 17α-ethinylestradiol when associated with nC60 aggregates during dietary exposure in adult male zebrafish (Danio rerio). Chemosphere 2010, 81, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Vale, G.; Franco, C.; Diniz, M.S.; dos Santos, M.M.C.; Domingos, R.F. Bioavailability of cadmium and biochemical responses on the freshwater bivalve Corbicula fluminea—The role of TiO2 nanoparticles. Ecotoxicol. Environ. Saf. 2014, 109, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, X.; Niu, Q.; Chen, Y.; Crittenden, J.C. Enhanced accumulation of arsenate in carp in the presence of titanium dioxide nanoparticles. Water. Air. Soil Pollut. 2007, 178, 245–254. [Google Scholar] [CrossRef]
- Al-Subiai, S.N.; Arlt, V.M.; Frickers, P.E.; Readman, J.W.; Stolpe, B.; Lead, J.R.; Moody, A.J.; Jha, A.N. Merging nano-genotoxicology with eco-genotoxicology: An integrated approach to determine interactive genotoxic and sub-lethal toxic effects of C 60 fullerenes and fluoranthene in marine mussels, Mytilus sp. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 745, 92–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krysanov, E.Y.; Demidova, T.B. The effect of low concentrations of nanocrystalline cerium dioxide on the embryotoxicity of doxorubicin for fish. Dokl. Biol. Sci. 2012, 443, 117–119. [Google Scholar] [CrossRef]
- Silveira, L.T.; Liberatore, A.M.A.; Koh, I.H.J.; Bizeto, M.A.; Camilo, F.F. Combined bactericidal activity of silver nanoparticles and hexadecylpyridinium salicylate ionic liquid. J. Nanoparticle Res. 2015, 17, 17. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Z.; Feng, M.; Wang, L.; Wang, Z. The effects of hydroxylated multiwalled carbon nanotubes on the toxicity of nickel to Daphnia magna under different pH levels. Environ. Toxicol. Chem. 2014, 33, 2522–2528. [Google Scholar] [CrossRef]
- Han, Z.X.; He, G.D.; Wang, J.H.; Lv, C.X. Interaction influence of Cd(II) and nano-TiO2 on aggregation and adsorption kinetics toward marine algae. Int. J. Green Nanotechnol. Biomed. 2011, 3, 229–237. [Google Scholar] [CrossRef]
- Glinski, A.; Liebel, S.; Pelletier, É.; Voigt, C.L.; Randi, M.A.F.; Campos, S.X.; Oliveira Ribeiro, C.A.; Filipak Neto, F. Toxicological interactions of silver nanoparticles and organochlorine pesticides in mouse peritoneal macrophages. Toxicol. Mech. Methods 2016, 26, 251–259. [Google Scholar] [CrossRef]
- Judy, J.D.; Kirby, J.K.; McLaughlin, M.J.; McNear, D.; Bertsch, P.M. Symbiosis between nitrogen-fixing bacteria and Medicago truncatula is not significantly affected by silver and silver sulfide nanomaterials. Environ. Pollut. 2016, 214, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Bergum, S.; Nilsen, E.W.; Olsen, A.J.; Salaberria, I.; Ciesielski, T.M.; Baczek, T.; Konieczna, L.; Salvenmoser, W.; Jenssen, B.M. The impact of TiO2 nanoparticles on uptake and toxicity of benzo(a)pyrene in the blue mussel (Mytilus edulis). Sci. Total Environ. 2015, 511, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Borggaard, O.K.; Holm, P.E.; Hansen, H.C.B.; Cedergreen, N. Toxicity and uptake of TRI- and dibutyltin in Daphnia magna in the absence and presence of nano-charcoal. Environ. Toxicol. Chem. 2011, 30, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yan, X.; Pu, Y.; Xiao, F.; Wang, D.; Yang, M. Risks of single-walled carbon nanotubes acting as contaminants-carriers: Potential release of phenanthrene in Japanese medaka (Oryzias latipes). Environ. Sci. Technol. 2013, 47, 4704–4710. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wan, B.; Guo, L.H.; Yang, Y.; Ren, X. Insight into the mechanisms of combined toxicity of single-walled carbon nanotubes and nickel ions in macrophages: Role of P2X7 receptor. Environ. Sci. Technol. 2016, 50, 12473–12483. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, C.; Fang, H.; Sun, L.; Zhang, H.; Feng, J.; Duan, D.; Liu, T.; Shi, J. Mitigation of Cu(Ii) Phytotoxicity to Rice (Oryza sativa) in the Presence of TiO2 and CeO2 Nanoparticles Combined with Humic Acid. Environ. Toxicol. Chem. 2015, 34, 1588–1596. [Google Scholar] [CrossRef]
- Hu, J.; Wang, D.; Wang, J.; Wang, J. Toxicity of lead on Ceriodaphnia dubia in the presence of nano-CeO2 and nano-TiO2. Chemosphere 2012, 89, 536–541. [Google Scholar] [CrossRef]
- Han, Z.; Li, J.; Bao, W.; Wang, J. Enhanced toxicity of atrazine to Daphnia magna in the presence of nano-CeO2. Chinese J. Geochemistry 2012, 31, 297–302. [Google Scholar] [CrossRef]
- McShan, D.; Zhang, Y.; Deng, H.; Ray, P.C.; Yu, H. Synergistic Antibacterial Effect of Silver Nanoparticles Combined with Ineffective Antibiotics on Drug Resistant Salmonella typhimurium DT104. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 369–384. [Google Scholar] [CrossRef]
- Fang, Q.; Shi, X.; Zhang, L.; Wang, Q.; Wang, X.; Guo, Y.; Zhou, B. Effect of titanium dioxide nanoparticles on the bioavailability, metabolism, and toxicity of pentachlorophenol in zebrafish larvae. J. Hazard. Mater. 2015, 283, 897–904. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Zhou, Q.; Lu, S.; Liu, R.; Cui, L.; Yin, D.; Mayer, P.; Jiang, G. Bioavailability of organochlorine compounds in aqueous suspensions of fullerene: Evaluated with medaka (Oryzias latipes) and negligible depletion solid-phase microextraction. Chemosphere 2010, 80, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wu, J.; Liu, M.; Zhu, G.; Chen, L.; Chang, Y.; Lu, H. Toxicity of binary mixtures of metal oxide nanoparticles to Nitrosomonas europaea. Chemosphere 2016, 153, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.W.; Li, Y.; Miao, A.J.; Yang, L.Y. Cd2+ toxicity as affected by bare TiO2 nanoparticles and their bulk counterpart. Ecotoxicol. Environ. Saf. 2012, 85, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Magesky, A.; Pelletier, É. Toxicity mechanisms of ionic silver and polymer-coated silver nanoparticles with interactions of functionalized carbon nanotubes on early development stages of sea urchin. Aquat. Toxicol. 2015, 167, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Anderson, T.A.; Acosta-Martinez, V.; Payton, P.; Cañas-Carrell, J.E. The influence of multiwalled carbon nanotubes on polycyclic aromatic hydrocarbon (PAH) bioavailability and toxicity to soil microbial communities in alfalfa rhizosphere. Ecotoxicol. Environ. Saf. 2015, 116, 143–149. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Sun, H.; Lu, G. Influence of multiwall carbon nanotubes on the toxicity of 17β-estradiol in the early life stages of zebrafish. Environ. Sci. Pollut. Res. 2018, 25, 7566–7574. [Google Scholar] [CrossRef]
- Hernández-Moreno, D.; Li, L.; Connolly, M.; Conde, E.; Fernández, M.; Schuster, M.; Navas, J.M.; Fernández-Cruz, M.L. Mechanisms underlying the enhancement of toxicity caused by the coincubation of zinc oxide and copper nanoparticles in a fish hepatoma cell line. Environ. Toxicol. Chem. 2016, 35, 2562–2570. [Google Scholar] [CrossRef]
- Schwab, F.; Bucheli, T.D.; Camenzuli, L.; Magrez, A.; Knauer, K.; Sigg, L.; Nowack, B. Diuron sorbed to carbon nanotubes exhibits enhanced toxicity to chlorella vulgaris. Environ. Sci. Technol. 2013, 47, 7012–7019. [Google Scholar] [CrossRef]
- Lahive, E.; Matzke, M.; Durenkamp, M.; Lawlor, A.J.; Thacker, S.A.; Pereira, M.G.; Spurgeon, D.J.; Unrine, J.M.; Svendsen, C.; Lofts, S. Sewage sludge treated with metal nanomaterials inhibits earthworm reproduction more strongly than sludge treated with metal metals in bulk/salt forms. Environ. Sci. Nano 2017, 4, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Li, S.; Qiao, J.; Wang, H.; Li, L. Synergistic effects of nano-sized titanium dioxide and zinc on the photosynthetic capacity and survival of Anabaena sp. Int. J. Mol. Sci. 2013, 14, 14395–14407. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, C. Fullerene nanoparticles affect the fate and uptake of trichloroethylene in phytoremediation systems. Environ. Eng. Sci. 2010, 27, 989–992. [Google Scholar] [CrossRef]
- Hu, X.; Kang, J.; Lu, K.; Zhou, R.; Mu, L.; Zhou, Q. Graphene oxide amplifies the phytotoxicity of arsenic in wheat. Sci. Rep. 2014, 4, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, J.; Salaberria, I.; Styrishave, B.; Staňková, R.; Ciesielski, T.M.; Olsen, A.J.; Posch, W.; Flaten, T.P.; Krøkje, Å.; Salvenmoser, W.; et al. Exposure of juvenile turbot (Scophthalmus maximus) to silver nanoparticles and 17α-ethinylestradiol mixtures: Implications for contaminant uptake and plasma steroid hormone levels. Environ. Pollut. 2017, 220, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Fan, W.H.; Wang, W.X. Role of titanium dioxide nanoparticles in the elevated uptake and retention of cadmium and zinc in Daphnia magna. Environ. Sci. Technol. 2012, 46, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Xia, X.; Zhai, Y.; Zhang, X.; Zhao, X.; Zhang, P. Influence of carbon nanotubes with preloaded and coexisting dissolved organic matter on the bioaccumulation of polycyclic aromatic hydrocarbons to Chironomus plumosus larvae in sediment. Environ. Toxicol. Chem. 2014, 33, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Azevedo Costa, C.L.; Chaves, I.S.; Ventura-Lima, J.; Ferreira, J.L.R.; Ferraz, L.; De Carvalho, L.M.H.; Monserrat, J.M. In vitro evaluation of co-exposure of arsenium and an organic nanomaterial (fullerene, C60) in zebrafish hepatocytes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012, 155, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.M.; Zha, J.M.; Shi, B.Y.; Wang, D.S.; Wang, Z.J.; Tang, H.X. In vivo toxicity of nano-C60 aggregates complex with atrazine to aquatic organisms. Chin. Sci. Bull. 2010, 55, 339–345. [Google Scholar] [CrossRef]
- Cano, A.M.; Maul, J.D.; Saed, M.; Irin, F.; Shah, S.A.; Green, M.J.; French, A.D.; Klein, D.M.; Crago, J.; Canas-Carrell, J.E. Trophic Transfer and Accumulation of Multiwalled Carbon Nanotubes in the Presence of Copper Ions in Daphnia magna and Fathead Minnow (Pimephales promelas). Environ. Sci. Technol. 2018, 52, 794–800. [Google Scholar] [CrossRef]
- Nunes, S.M.; Josende, M.E.; Ruas, C.P.; Gelesky, M.A.; Júnior, F.M.R.d.S.; Fattorini, D.; Regoli, F.; Monserrat, J.M.; Ventura-Lima, J. Biochemical responses induced by co-exposition to arsenic and titanium dioxide nanoparticles in the estuarine polychaete Laeonereis acuta. Toxicology 2017, 376, 51–58. [Google Scholar] [CrossRef]
- Ko, K.S.; Koh, D.C.; Kong, I.C. Toxicity evaluation of individual and mixtures of nanoparticles based on algal chlorophyll content and cell count. Materials 2018, 11, 121. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Zhang, Z.; Lv, M.; Song, W.; Lv, Y. Toxic effects of nanomaterial-adsorbed cadmium on Daphnia magna. Ecotoxicol. Environ. Saf. 2018, 148, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wu, J.; Liu, M.; Chen, L.; Zhu, G.; Lu, H. Physiological and transcriptional responses of Nitrosomonas europaea to TiO2 and ZnO nanoparticles and their mixtures. Environ. Sci. Pollut. Res. 2016, 23, 13023–13034. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, X.; Zhang, Z.; Chen, Y.; Crittenden, J.C. Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite. Environ. Pollut. 2009, 157, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Molins-Delgado, D.; Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. Single and joint ecotoxicity data estimation of organic UV filters and nanomaterials toward selected aquatic organisms. Urban groundwater risk assessment. Environ. Res. 2016, 145, 126–134. [Google Scholar] [CrossRef]
- Ye, N.; Wang, Z.; Fang, H.; Wang, S.; Zhang, F. Combined ecotoxicity of binary zinc oxide and copper oxide nanoparticles to Scenedesmus obliquus. J. Environ. Sci. Health Part A Toxic/Hazardous Subst. Environ. Eng. 2017, 52, 555–560. [Google Scholar] [CrossRef]
- Liu, Y.; Baas, J.; Peijnenburg, W.J.G.M.; Vijver, M.G. Evaluating the Combined Toxicity of Cu and ZnO Nanoparticles: Utility of the Concept of Additivity and a Nested Experimental Design. Environ. Sci. Technol. 2016, 50, 5328–5337. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeldt, R.R.; Seitz, F.; Zubrod, J.P.; Feckler, A.; Merkel, T.; Lüderwald, S.; Bundschuh, R.; Schulz, R.; Bundschuh, M. Does the presence of titanium dioxide nanoparticles reduce copper toxicity? A factorial approach with the benthic amphipod Gammarus fossarum. Aquat. Toxicol. 2015, 165, 154–159. [Google Scholar] [CrossRef]
- Jośko, I.; Oleszczuk, P.; Skwarek, E. Toxicity of combined mixtures of nanoparticles to plants. J. Hazard. Mater. 2017, 331, 200–209. [Google Scholar] [CrossRef]
- Yang, X.Y.; Edelmann, R.E.; Oris, J.T. Suspended C60 nanoparticles protect against short-term UV and fluoranthene photo-induced toxicity, but cause long-term cellular damage in Daphnia magna. Aquat. Toxicol. 2010, 100, 202–210. [Google Scholar] [CrossRef]
- Park, J.W.; Henry, T.B.; Ard, S.; Menn, F.M.; Compton, R.N.; Sayler, G.S. The association between nC 60 and 17α-ethinylestradiol (EE2) decreases EE2 bioavailability in zebrafish and alters nanoaggregate characteristics. Nanotoxicology 2011, 5, 406–416. [Google Scholar] [CrossRef]
- Xia, X.; Chen, X.; Zhao, X.; Chen, H.; Shen, M. Effects of carbon nanotubes, chars, and ash on bioaccumulation of perfluorochemicals by chironomus plumosus larvae in sediment. Environ. Sci. Technol. 2012, 46, 12467–12475. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Xia, X.; Wang, F.; Zhang, P.; Zhao, X. Influences of multiwalled carbon nanotubes and plant residue chars on bioaccumulation of polycyclic aromatic hydrocarbons by Chironomus plumosus larvae in sediment. Environ. Toxicol. Chem. 2012, 31, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; He, Y.; Fortner, J.D.; Chen, Y.; Hughes, J.B. Effects of aqueous stable fullerene nanocrystal (nC60) on copper (trace necessary nutrient metal): Enhanced toxicity and accumulation of copper in Daphnia magna. Chemosphere 2013, 92, 1245–1252. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, S.; Wang, Z.; Ye, N.; Fang, H. Algal toxicity of binary mixtures of zinc oxide nanoparticles and tetrabromobisphenol A: Roles of dissolved organic matters. Environ. Toxicol. Pharmacol. 2018, 64, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, P.L.; Chandler, G.T.; Templeton, R.C.; Demarco, A.; Scrivens, W.A.; Englehart, B.A. Influence of sediment—Amendment with single-walled carbon nanotubes and diesel soot on bioaccumulation of hydrophobic organic contaminants by benthic invertebrates. Environ. Sci. Technol. 2008, 42, 3879–3885. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, J.; Cai, Z. TiO2 nanoparticles in the marine environment: Impact on the toxicity of tributyltin to abalone (Haliotis diversicolor supertexta) embryos. Environ. Sci. Technol. 2011, 45, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.G.; Wang, W.X. Influences of ambient carbon nanotubes on toxic metals accumulation in Daphnia magna. Water Res. 2013, 47, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Trinh, X.T.; Choi, J.S.; Jeon, H.; Byun, H.G.; Yoon, T.H.; Kim, J. Quasi-SMILES-Based Nano-Quantitative Structure–Activity Relationship Model to Predict the Cytotoxicity of Multiwalled Carbon Nanotubes to Human Lung Cells. Chem. Res. Toxicol. 2018, 31, 183–190. [Google Scholar] [CrossRef]
- Choi, J.-S.; Ha, K.M.; Trinh, X.T.; Yoon, T.H.; Byun, H.G. Towards a generalized toxicity prediction model for metal oxide nanomaterials using integrated data from different sources. Sci. Rep. 2018, 8, 6110. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Trinh, T.X.; Yoon, T.H.; Kim, J.; Byun, H.G. Quasi-QSAR for predicting the cell viability of human lung and skin cells exposed to different metal oxide nanomaterials. Chemosphere 2019, 217, 243–249. [Google Scholar] [CrossRef]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.X.; Ha, M.K.; Choi, J.S.; Byun, H.G.; Yoon, T.H. Curation of datasets, assessment of their quality and completeness, and nanoSAR classification model development for metallic nanoparticles. Environ. Sci. Nano 2018, 5, 1902–1910. [Google Scholar] [CrossRef]
- Burk, J.; Sikk, L.; Burk, P.; Manshian, B.B.; Soenen, S.J.; Scott-Fordsmand, J.J.; Tamm, T.; Tämm, K. Fe-Doped ZnO nanoparticle toxicity: Assessment by a new generation of nanodescriptors. Nanoscale 2018, 10, 21985–21993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tämm, K.; Sikk, L.; Burk, J.; Rallo, R.; Pokhrel, S.; Mädler, L.; Scott-Fordsmand, J.J.; Burk, P.; Tamm, T. Parametrization of nanoparticles: Development of full-particle nanodescriptors. Nanoscale 2016, 8, 16243–16250. [Google Scholar] [CrossRef] [PubMed]
- Économiques, O. De Coopération et de Développement Guidance Document on the Validation of (Quantitative) Structure-Activity Relationship [(Q)Sar] Models; OECD: Paris, France, 2007; Volume 2, pp. 1–154. [CrossRef]
- Raunio, H. In silico toxicology non-testing methods. Front. Pharmacol. 2011, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Économiques, O. De Coopération et de Développement Test No. 211: Daphnia magna Reproduction Test; OECD: Paris, France, 2012. [CrossRef]
- Économiques, O. De Coopération et de Développement Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013; pp. 1–22. [CrossRef]
- OECD Test Guideline No. 212: Fish, Short-term Toxicity Test on Embryo and Sac-fry Stages. Organisation for Economic Cooperation and Development; OECD: Paris, France, 1998; Volume 212, pp. 1–20. [CrossRef]
- Vinken, M. Omics-based input and output in the development and use of adverse outcome pathways. Curr. Opin. Toxicol. 2019, 18, 8–12. [Google Scholar] [CrossRef]



| No. | Reference | Nano-Mixture | Test System | Descriptors | Endpoint | Algorithm | Equations | No. Data | R2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mikolajczyk et al., 2016 [12] | TiO2 NPs + Au/Pd | None | 27 structural descriptors | Photocatalytic activity | Multiple linear regression | 17 | 0.80–0.89 | |
| 2 | Mikolajczyk et al., 2018 [11] | TiO2 NPs + Au/Ag/Pt | Chinese hamster ovary cell line | 5 quantum descriptors | Effective concentration (EC50) | Multiple linear regression | 26 | 0.83–0.94 | |
| 3 | Mikolajczyk et al., 2019 [10] | TiO2 NPs + Au/Ag/Pt/Pd | Chinese hamster ovary cell line | 9 quantum descriptors | Effective concentration (EC50) | Multiple linear regression | 29 | 0.80–0.87 | |
| Decision tree | None | 29 | 0.74–0.90 | ||||||
| Photocatalytic activity | Decision tree | None | 29 | 0.80–0.82 |
| No. | Article | Nanomaterials | Mixed Substance | Mixed Substance Type | Toxicity Test Guideline | Toxicity Endpoint | Mixture Toxicity Models |
|---|---|---|---|---|---|---|---|
| 1 | Azevedo et al., 2017 Science of the Total Environment [14] | ZnO NPs | Ag NPs | Nanometal | OECD 202; OECD 211 | Immobilization, Reproduction | CA and IA |
| 2 | Baun et al., 2008 Aquatic Toxicology [98] | C60 fullerenes | Atrazine, Methylparathione, Pentachlorophenol, Phenanthrene | Organic compounds | OECD 211, ISO 6341 | Reproduction, Immobilization, Bioaccumulation | None |
| 3 | Brausch et al., 2010 Environmental Toxicology and Chemistry [97] | C60 fullerenes | Bifenthrin, Tribufos | Organic compounds | EPA-821-R-02-012 | Mortality, Reproduction, Growth inhibition | None |
| 4 | Cano et al., 2018 Environmental Science and Technology [178] | MWCNT | Cu2+ | Metal ions | None | Metallothionein inhibition, Bioaccumulation | None |
| 5 | Fan et al., 2011 Environmental Pollution [107] | TiO2 NPs | Cu2+ | Metal ions | None | Metallothionein inhibition, Mortality, Bioaccumulation | None |
| 6 | Fan et al., 2012 Journal of Nanomaterials [106] | TiO2 NPs | Cu2+ | Metal ions | None | Oxidative stress, Metal-ATPase activity, Mortality | None |
| 7 | Fang et al., 2011 Environmental Toxicology and Chemistry [153] | Nano-charcoal | Tributyltin, Dibutyltin | Organometallic | ISO 6341 | Reproduction, Immobilization, Bioaccumulation | None |
| 8 | Gao et al., 2018 Ecotoxicology and Environmental Safety [181] | MnO2 NPs, Nano-hydroxyapatite | Cd2+ | Metal ions | OECD 202 | Immobilization, Oxidative stress | None |
| 9 | Han et al., 2012 Chinese Journal of Geochemistry [158] | CeO2 NPs | Atrazine | Organic compounds | US. EPA 2001 | Reproduction, Mortality, Bioaccumulation | None |
| 10 | Hartmann et al., 2012 Aquatic Toxicology [114] | TiO2 NPs | Cu2+ | Metal ions | OECD 202, ISO 6341 | Bioaccumulation | None |
| 11 | Kim et al., 2009 Environmental Science & Technology [136] | MWCNT | Cu2+ | Metal ions | None | Bioaccumulation, Mortality, Oxidative stress | None |
| 12 | Kim et al., 2010 Environmental Toxicology and Chemistry [73] | Cu NPs | SWCNT | Nanocarbon | None | Mortality, Bioaccumulation | None |
| 13 | Lopes et al., 2016 J. Hazardous Materials [13] | Ag NPs, ZnO NPs | Ag NPs, ZnO NPs, Ag+, Zn2+ | Nano-oxide, Metal ions | OECD 202 | Immobilization | CA and IA |
| 14 | Martín-de-Lucía et al., 2019 Science of The Total Environment [16] | Graphite-diamond | Fungicide thiabendazole | Organic compounds | OECD 202 | Immobilization | CA and IA |
| 15 | Molins-Delgado et al., 2016 Environmental Research [184] | Ag NPs | Benzophenone, Ethyl-PABA, 4-methylbenzylidene camphor, Ethylhexyl-methoxy cinamate | Organic compounds | ISO 6341 | Immobilization | None |
| 16 | Park et al., 2019 Journal of Nanoparticle Research [117] | TiO2 NPs, ZnO NPs | Ag+ | Metal ions | OECD 202 | Immobilization | None |
| 17 | Park et al., 2019 Molecular & Cellular Toxicology [81] | Fe3O4 NPs | Zn2+ | Metal ions | OECD 202 | Immobilization | CA and IA |
| 18 | Rosenfeldt et al., 2014 Environmental Science & Technology [112] | TiO2 NPs | Cu2+, Ag+, As5+ | Metal ions | OECD 202 | Bioaccumulation, Immobilization | None |
| 19 | Rosenfeldt et al., 2015 Environmental Science & Technology [113] | TiO2 NPs | Cu2+ | Metal ions | OECD 202 | Bioaccumulation, Immobilization | None |
| 20 | Sanchis et al., 2016 Environmental Science & Technology [59] | C60 fullerenes | Nonylphenol, Triclosan, Malathion, Diuron, Glyphosate | Organic compounds | OECD 202, ISO 6341 | Immobilization | None |
| 21 | Seitz et al., 2012 Environmental Toxicology and Chemistry [110] | TiO2 NPs | As5+ | Metal ions | OECD 202 | Immobilization | None |
| 22 | Tan and Wang, 2014 Environmental Pollution [109] | TiO2 NPs | Cd2+, Zn2+ | Metal ions | None | Oxidative stress, Uptake, Retention of dietary, Mortality | None |
| 23 | Tan et al., 2012 Environmental Science & Technology [174] | TiO2 NPs | Cd2+, Zn2+ | Metal ions | None | Uptake, Bioaccumulation, Retention of dietary | None |
| 24 | Tao et al., 2013 Chemosphere [193] | C60 fullerenes | Cu2+ | Metal ions | US EPA 2024 | Metal ATPase activity, Mortality, Bioaccumulation | None |
| 25 | Tian et al., 2014 Advanced Materials Research [108] | TiO2 NPs | Penta-brominated diphenyl ether | Organic compounds | OECD 202 | Immobilization, Mortality | None |
| 26 | Vega et al., 2019 Ecotoxicology and Environmental Safety [65] | TiO2 NPs | Organic UV filter oxybenzone, Benzylparaben | Organic compounds | ISO 6341 | Immobilization | None |
| 27 | Wang et al., 2014 Environmental Toxicology and Chemistry [148] | MWCNT | Ni2+ | Metal ions | None | Immobilization, Bioaccumulation | None |
| 28 | Wang et al., 2016 Environmental Pollution [34] | MWCNT, SWCNT | Cd2+ | Metal ions | OECD 202 | Mortality, Bioaccumulation | None |
| 29 | Ye et al., 2018 Nanotoxicology [18] | ZnO NPs | GO NPs | Nanocarbon | OECD 201, 202, 236 | Growth inhibition, Immobilization, Mortality, Oxidative stress | None |
| 30 | Yan et al., 2010 Chinese Science Bulletin [177] | C60 fullerenes | Atrazine | Organic compounds | None | Reproduction, Deformity rate, Hatching rate | None |
| 31 | Yang et al., 2010 Aquatic Toxicology [189] | C60 fullerenes | Fluoranthene | Organic compounds | EPA 6004-90027 | Immobilization, Cell damage | None |
| 32 | Ye et al., 2018 Nanotoxicology [18] | ZnO NPs | GO NPs | Nanocarbon | OECD 201, 202, 236 | Growth inhibition, Mortality, Immobilization | None |
| 33 | Yu & Wang, 2013 Water Research [197] | MWCNT, SWCNT | Cd2+, Zn2+ | Metal ions | None | Uptake, Bioaccumulation | None |
| 34 | Yu & Wang, 2014 Environmental Toxicology and Chemistry [26] | C60 fullerenes | Cd2+, Zn2+ | Metal ions | None | Bioaccumulation | None |
| 35 | Zhang et al., 2015 Journal of Environmental Sciences [31] | Ag NPs | Ag+ | Metal ions | OECD 202 | Mortality | None |
| No. | Article | Nanomaterials | Mixed Substance | Mixed Substance Type | Toxicity Test Guideline | Toxicity Endpoint | Mixture Toxicity Models |
|---|---|---|---|---|---|---|---|
| 1 | Azevedo Costa et al., 2012 Comparative Biochemistry and Physiology, Part C [176] | C60 fullerenes | As3+ | Metal ions | None | Cell viability, Mitochondrial dehydrogenase functionality, Oxidative stress, Bioaccumulation | None |
| 2 | Fang et al., 2015 Journal of Hazardous Materials [160] | TiO2 NPs | Pentachlorophenol | Organic compounds | None | Bioaccumulation, Oxidative stress, Gene expression, Glutathione level | None |
| 3 | Ferreira et al., 2014 Aquatic Toxicology [131] | C60 fullerenes | Benzo[a]pyrene | Organic compounds | None | Cell viability, Oxidative stress, Bioaccumulation, Glutathione level | None |
| 4 | Ginzburg et al., 2018 ACS Nano [52] | Au NPs | Polysorbate 20 | Organic compounds | None | Mortality | None |
| 5 | Henry et al., 2007 Environmental Health Perspectives [56] | C60 fullerenes | Tetrahydrofuran | Organic compounds | None | Mortality, Gene expression | None |
| 6 | Henry et al., 2013 Environmental Science & Technology [36] | C60 fullerenes | Hg+ | Metal ions | Plymouth University | Mortality, Bioaccumulation, Gene expression | None |
| 7 | Hu et al., 2011 Environmental Pollution [38] | TiO2 NPs | Cd2+ | Metal ions | None | Bioaccumulation | None |
| 8 | Hua et al., 2016 NanoImpact [74] | TiO2 NPs | ZnO NPs | Nano-oxide | OECD 157 | Mortality | RA and CA |
| 9 | Krysanov & Demidova, 2012 Doklady Biological Sciences [146] | CeO2 NPs | Doxorubicin | Organic compounds | None | Malformations, Hatching rate | None |
| 10 | Miao, 2015 Aquatic Toxicology [119] | TiO2 NPs | Pb2+ | Metal ions | None | Gene expression, Locomotion activity, Thyroid hormone content, Bioaccumulation | None |
| 11 | Park et al., 2011 Nanotoxicolog y [190] | C60 fullerenes | 17α-Ethinyl-estradiol | Organic compounds | None | Bioaccumulation, Gene expression | None |
| 12 | Park et al., 2015 Molecular & Cellular Toxicology [60] | Ag NPs | Ag nanotube | Nanometal | None | Gene expression | None |
| 13 | Pavagadhi et al., 2014 Water Research [77] | Ag NPs, TiO2 NPs | Ni2+, Mg2+, Zn2+, Cu2+, Cd2+, Fe2+, Cr3+, Hg2+, As5+, Al3+, Pb2+, Mn2+ | Metal ions | None | Mortality, Heartbeat, Hatching rate, Uptake | None |
| 14 | Wang et al., 2014 Nanotoxicology [84] | TiO2 NPs | Decabromdiphenyl ether | Organic compounds | None | Bioaccumulation, Locomotion activity, Oxidative stress, Gene expression | None |
| 15 | Ye et al., 2018 Nanotoxicology [18] | ZnO NPs | GO NPs | Nanocarbon | OECD 236 | Mortality, Oxidative stress | None |
| 16 | Yan et al., 2014 Nanoscale Research Letters [103] | TiO2 NPs | Bis-Phenol A | Organic compounds | OECD 212 | Hatching rate, Immobilization, Heart sac edema, Abnormality rate | None |
| 17 | Yan et al., 2018 Environmental Science and Pollution Research [166] | MWCNT | 17 β -estradiol | Organic compounds | None | Mortality, Hatching rate, Abnormality rate | None |
| 18 | Zhang et al., 2012 Environmental Toxicology and Chemistry [140] | QDs (CdSe) | Cu2+ | Metal ions | None | Mortality, Malformations, Hatching rate | None |
| No. | Article | Nanomaterials | Mixed Substance | Mixed Substance Type | Toxicity Test Guideline | Toxicity Endpoint | Mixture Toxicity Models |
|---|---|---|---|---|---|---|---|
| 1 | Cuahtecontzi-Delint et al., 2013 International Journal of Chemical Reactor Engineering [138] | CeO2 NPs | Surfactants (Tween 80, Triton X114, and Polyvinyl Pyrrolidone) | Organic compounds | None | Growth inhibition | None |
| 2 | Li et al., 2005 Nanotechnology [134] | Ag NPs | Antibiotics | Organic compounds | None | Growth inhibition | None |
| 3 | Santaella et al., 2014 Environmental Science & Technology [55] | TiO2 NPs | Cd2+ | Metal ions | None | Cell viability, Oxidative stress | None |
| 4 | Shahverdi et al., 2007 Nanomedicine Nanotechnology, Biology and Medicine [129] | Ag NPs | Antibiotics | Organic compounds | None | Growth inhibition | None |
| 5 | Silveira et al., 2015 Journal of Nanoparticle Research [147] | Ag NPs | Hexadecylpyridinium salicylate | Organic compounds | None | Growth inhibition | None |
| 6 | Srivastava et al., 2016 Journal of Environmental Sciences [133] | ZnO NPs | TiO2 NPs | Nano-oxide | None | Cell wall damage, Growth inhibition | None |
| 7 | Tong et al., 2015 Environmental Science & Technology [120] | ZnO NPs | TiO2 NPs | Nano-oxide | None | Photoproduction, ATP production, Oxidative stress, Bioaccumulation | None |
| 8 | Wilke et al., 2016 Environmental Science and Technology [57] | TiO2 NPs | Ag NPs | Nanometal | None | ATP production | None |
| 9 | Zhang et al., 2009 Environmental Science & Technology [37] | C60 fullerenes | Tetrahydrofuran (THF) | Organic compounds | None | Growth inhibition, Indigo degradation | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, T.X.; Kim, J. Status Quo in Data Availability and Predictive Models of Nano-Mixture Toxicity. Nanomaterials 2021, 11, 124. https://doi.org/10.3390/nano11010124
Trinh TX, Kim J. Status Quo in Data Availability and Predictive Models of Nano-Mixture Toxicity. Nanomaterials. 2021; 11(1):124. https://doi.org/10.3390/nano11010124
Chicago/Turabian StyleTrinh, Tung X., and Jongwoon Kim. 2021. "Status Quo in Data Availability and Predictive Models of Nano-Mixture Toxicity" Nanomaterials 11, no. 1: 124. https://doi.org/10.3390/nano11010124