1. Introduction
Despite the extensive production and use of nanoproducts, knowledge concerning the safety of their handling and use is lagging, and no biomarkers are currently used to monitor exposure in practice.
Several studies have searched for the most useful biomarkers of oxidative damage in humans [
1,
2,
3,
4,
5], and it has been shown that no single marker may be satisfactory [
6,
7]. The need for nanotoxicology studies in humans is considered vital due to the potential damage to cells, organs, and organisms, and to a lack of human data, especially in occupational settings [
8,
9], where the exposure is the highest. Consumer product usage, such as sunscreen, may also be cause for concern, as absorption of nanoparticles through the skin was found [
10,
11]. The experimental data show that cell membranes with lipid bilayers are the primary target for nanoparticle attack, which may ultimately lead to cell death [
12]. It has been shown that nanoparticle-induced oxidative stress and reactive oxygen species (ROS) produced can lead to inflammation, apoptosis, and genotoxicity. Additionally, a high accumulation of nanoparticles on the cell surface is a further trigger of cytotoxicity. Moreover, chronic, even low-dose exposures may induce more subtle changes and lead to disease initiation or progression [
12]. Particularly, little is known about more complex mixtures of nanoparticles and their interactions with other factors, such as diet or physical load.
In our previous study in 2016, we have described and characterized, in detail, the workplace aerosols formed in the course of the production and handling of nanocomposites during all three main operations in workshops (welding, smelting, and machining) [
13,
14]. Eleven in vivo markers of oxidative stress and 12 markers of inflammation were analyzed in the exhaled breath condensate (EBC) of workers in the nanocomposites research plant [
13,
14]. Significant increases in the majority of pre-shift and post-shift markers of oxidation of lipids, nucleic acids and proteins in the EBC of workers were observed, compared to control subjects’ samples. Moreover, spirometry showed impairment in post-shift lung function.
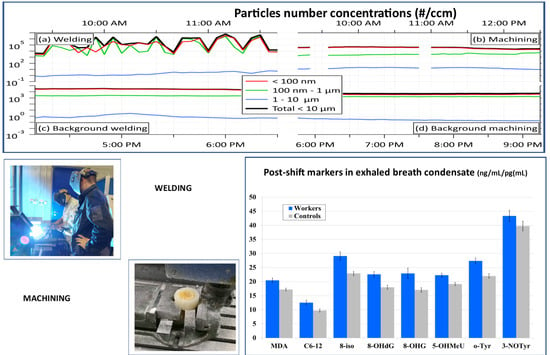
For this follow-up study in 2017 and 2018, eight markers of oxidative stress were selected, including biomarkers derived from free radical oxidation of polyunsaturated fatty acids malondialdehyde (MDA), aldehydes C6–C12 (i.e., hexanal (C6), heptanal (C7), octanal (C8), nonanal (C9), decanal (C10), undecanal (C11) and dodecanal (C12)), and 8-iso-prostaglandin F2α (8-isoprostane). Markers of oxidation of nucleic acid bases, including 8-hydroxy-2-deoxyguanosine (8-OHdG), 8-hydroxyguanosine (8-OHG), and 5-hydroxymethyl uracil (5-OHMeU), were examined. Markers of oxidative stress related to proteins, including o-tyrosine (o-Tyr) and 3-nitrotyrosine (3-NOTyr), were examined.
To improve and enlarge the pilot study from 2016, we extended the analysis of markers to plasma and urine in the following two years, and, to the best of our knowledge, no study has yet focused on the analysis of oxidative stress markers across several bodily fluids of workers handling nanomaterials.
The control subjects in 2016 and 2017 provided samples in the morning only, whereas, in 2018, the control subjects’ examination was enriched by a second sampling in the afternoon, at a comparable time of the post-shift samples collection in the workers.
The first aim of this study was to find out whether ongoing exposure to workplace aerosol, with a relatively stable proportion of ultrafine particles under similar conditions, would show an increasing trend, due to a potentially cumulative effect, or a negative trend, due to a potential adaptation to chronic exposure. To achieve this, we have also compared the results to those from the subgroup of workers who participated repeatedly and who provided their EBC samples in all three years (2016, 2017, and 2018). Here, we focused both on chronic effects, using pre-shift sample collection, and the acute effects related to the last shift exposure, as both effects were observed in 2016.
Our second aim was to verify the practical usefulness of the noninvasive method of EBC collection successfully used in 2016 and to compare the results of the same markers, collected and analyzed in parallel, in plasma and urine over the two following years, 2017 and 2018. This aim was selected due to concerns about the methodology related to dilution of the EBC fluid, for which no parameter exists to control concentration [
15].
Our third aim was to select the most representative fluids and markers for bio-monitoring of the workers handling nanomaterials in order to monitor exposure and prevent unwanted late effects from appearing in experimental studies.
4. Discussion
It has been repeatedly declared that there is an urgent need to find appropriate biomarkers to control the level of oxidative stress in the most-exposed human subjects in order to prevent potentially severe consequences [
25,
26,
27]. However, data on the markers of oxidative stress in the literature are scarce.
Our first study describing the data from this research plant in 2016 has proven that occupational exposure was the primary cause of the elevation in the concentration of the biomarkers of oxidative stress in the EBC, both pre-shift, related to the chronic exposure, and post-shift, related to the impact of the exposure during the monitoring day.
The new results presented here, for both pre-shift and post-shift measurements, showed a similar trend in both follow-up years in all biological fluids, even if significance has not been reached for some markers. This may be related to the limited number of subjects available for examination.
This study examined the control subjects in 2018 twice, at two different times, and found new, rather unexpected results in the afternoon samples, where the levels of 50% of total markers increased. Despite this elevation, the results for the workers in 2018 were significantly higher in 100% of these findings, compared to those of the control subjects. The findings led us to analyze a set of 20 non-occupational parameters and environmental contamination data in order to explain this observation.
The level of workplace aerosol exposure measured by objective methods was relatively high in all three years, as was the proportion of particles under 100 nm.
In this study, we attempted to compare the effect of acute exposure of workers in different working processes, where, for all three years, only EBC results were available. In 2016, only 37.5% of EBC markers were elevated post-shift when the shift included three operations: welding and smelting in workshop 1 and machining in workshop 2. In 2017, the proportion of elevated markers increased to 62.5%, when all workers performed machining. In the final year (2018), exposure consisted of welding in workshop 1 and machining in workshop 2, and the elevation was observed for 100% of EBC samples. MDA appeared to be the most responsive marker, as it elevated in the post-shift EBC every year.
If we compare the aerosol exposure in these three years, we find that the highest exposure to all nanoparticles during the shift was observed in 2016, significantly lower in 2017 and again lower in 2018, both when we use mass concentrations and when we use number concentrations (
Table 4) as the exposure metrics. This observation goes against the trend in biomarkers concentration found in EBC in the years 2017 and 2018 (
Figure 1 and
Figure 2).
On the other hand, if we compare the mass concentrations summed up in all of the years by SEM/EDS analyzed elements, determined at machining—the only working operation used in all three years—the exposure increased between the years 2016, 2017 and 2018, respectively, in the ratio of 1, 4:5, 5:8 (as shown in
Table 3), respectively, in accordance with the increasing trend in biomarkers found in EBC in the last two years. As already mentioned, these elements were largely emitted by machining operations with origin from the samples and machines. On the other side, the total mass concentrations, determined by gravimetry on the same samples, contained many inorganic salts and organic material, including those coming from outdoors.
Accordingly, in another study by Ricelli et al., EBC levels of MDA were elevated post-8 h occupational exposure to stainless steel tungsten inert gas welding fumes, which contained Cr and Ni. In addition, the EBC Cr concentration was higher post-shift (0.08 μg/L) than pre-shift (0.06 μg/L), confirming the absorption of Cr due to inhalation exposure [
28].
In a study of fire-fighters by Wu et al., there was a marginal increase of 8-isoprostane levels in the post-shift EBC samples on burn days (3.5–3.8 pg/mL;
p = 0.06) compared to non-burn days (2.86–3.20 pg/mL) [
29]. These post-shift levels were approximately 10-fold lower than the concentrations in this study’s worker subjects post-shift, as a less sensitive method, the enzyme-linked immunosorbent assays (ELISA), was used in that study.
The chronic effect in our workers, evaluated by the pre-shift EBC samples, slowly decreased, from 87.5% in 2016 to 62.5% in 2017, and to 37.5% in 2018. Among the eight markers examined, only 8-OHdG concentration was elevated pre-shift in all three years. As the usual daily exposure time reported by the workers has not shortened, this may be explained by the fact that the total average duration of exposure in years was lower in 2017 and 2018. However, this difference did not reach significance. We also speculate that the workers may have behaved more cautiously, as a reflection of the results of our study from 2016. Anyway, the personnel respiratory protective equipment has not been used in any year.
Similarly, in the above-mentioned occupational study focused on nanoparticles, a chronic/subacute effect on the EBC MDA concentrations was observed due to exposures to tungsten inert welding fumes. The pre-shift MDA levels were higher on Friday, i.e., at the end of the week, compared to the pre-shift sample on Monday [
28].
The length of exposure, and the time lag from the end of exposure to sample collection, may be important in the evaluation of post-exposure samples. In our recent study, the samples were collected within 1 h after 2.5–3.5 h exposure; however, other studies show that the levels of the markers can further rise, becoming even more significantly different.
In the study by Graczyk et al., which measured 8-OHdG levels in the plasma and urine, after 60 min lasting exposure to tungsten inert gas welding fumes, marker elevations were not seen 1 h post-exposure but appeared at 3 h post-exposure. These authors recommended analysis at even later time points of 12 h and 24 h post-exposure [
30].
In the report by Riccelli et al., the EBC collections after 8 h shift exposure showed an increased level of MDA [
28]. Similarly, our earlier studies found the elevation in EBC markers concentrations for the workers exposed to metal oxide nanoparticles who worked 8 h shifts (samples were collected immediately following shifts) [
31,
32,
33]. In both EBC and urine (but not plasma, due to a noninvasive approach being desired), markers of oxidative stress were measured in nano-Fe-oxides exposed workers. Despite observing an increase in the levels of the EBC markers, the difference in the urine markers concentrations was less significant. Urine markers, however, showed the same trend, and some correlated with the EBC levels for markers such as MDA and C6-C12 [
21]. Similarly, the nano-TiO
2 exposed workers with higher exposure also had elevated urine markers concentrations [
32]. It is plausible that the elevation of urine markers levels may be dose-related, as these were not elevated in the subjects with significantly lower exposure, such as office employees of nano-TiO
2 manufacturing plants, despite the significantly higher concentrations of markers of oxidation of nucleic acids, proteins [
34] and lipids in the EBC [
35].
The patients with silicosis and asbestos-induced diseases, with carcinogenic silica or asbestos persisting in their lungs, displayed elevated EBC markers and plasma and/or urine markers levels, even decades after their exposure ceased [
36]. The mechanism of oxidative stress is the key point and may be a concern regarding nanoparticles’ exposure.
The type and route of exposure are also important. The elevation of oxidative stress markers in the EBC, plasma and urine after UV irradiation in the solarium was observed after just 3 h. The skin irradiation of 80% of the body also resulted in a pronounced systemic effect [
37].
The regular working operations of the workers in the workshops were identical throughout all years. On the other hand, the operations selected for the monitoring day changed, which may have contributed to the differences related to individual markers. In addition, over the years, slightly different samples were selected for machining on the monitoring day, based on how the scientific interests of researchers have changed. This probably also affected the proportion of the analyzed elements in the working atmosphere. Obviously, not only the quantity and proportion of nanoparticles but the chemical composition of the aerosol may also play a role, especially Fe [
31].
When we merged the results of the biomarkers in workers performing welding in 2016 and 2018 and compared the levels with the results of the workers who performed machining, the results did not differ significantly in any of the eight monitored markers of oxidative stress. This may mean that higher numbers of subjects are needed to find differences. A difference was noticed in our pilot cytogenetic study; specifically, an increase of chromosomal breaks in a subgroup of researchers performing welding and smelting in 2016 [
38]. Therefore, in our recent research project (No. GACR 18-02079S of the Czech Science Foundation), we continue the follow-up of the workers to evaluate potential genetic and epigenetic changes [
39].
The accuracy of the EBC collection and analysis has been repeatedly questioned with regard to its reliability, as there is no marker available to assess its dilution that is as adequate as creatinine in urine [
15]. However, regardless of the absence of such a marker, an identical trend can be seen across
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6 (i.e., in the levels of both pre-shift and post-shift markers in the workers in all fluids examined, compared with the control subjects). These trends confirm the value of EBC marker analysis and are consistent with our earlier studies.
A systematic review by Shoman et al. and meta-analysis of EBC 8-isoprostane by immunological methods resulted in the determination of a reference level, with geometric mean 7.67 pg/mL (95% confidence interval 5.58–9.76) [
1], which is lower compared with our average value of 21 pg/mL in the control subjects and 26 pg/mL in the workers pre-shift.
The healthy adults had 8-OHdG levels 3.9 ng/mg creatinine (interquartile range 3–5.5 ng/mg creatinine) in the urine according to a meta-analysis of the studies by Graille et al. and LC-ESI-MS/MS analysis was superior to immunological methods, due to its higher sensitivity and specificity [
3]. Our control subjects had an average level of 8-OHdG, approximately 0.3 ng/mg creatinine, and no results were below the LOD [
30].
Unfortunately, results from human studies focusing on the markers of oxidation of lipids, nucleic acids and proteins show great variability. They are additionally complicated by the expression of results in different gravimetric and molar units. This occurs especially for urine samples, where several combinations of units may be used. For better comparison, we present the results of our analyses in EBC, plasma and urine in the groups of control subjects and workers in different variants of gravimetric and molar units in
Tables S1–S6.
A detailed analysis of potential correlations of the markers of oxidation with individual factors related to personal history, diseases, all types of medication, habits, and lifestyle was carried out.
As shown in
Table 1, the age of the workers throughout the years 2016–2018 did not differ. No correlation with age was seen in the markers in all fluids studied, similar to our earlier studies in exposed workers. Accordingly, in the EBC, no influence of age, gender and BMI was found by Shoman et al. in a recent meta-analysis of studies investigating 8-isoprostane, a marker of oxidation of lipids in the EBC [
1].
For the plasma marker levels, however, some studies show unequivocal results concerning the influence of age and/or gender. In the recent study by Pinchuk et al., 3-NOTyr was independent of these parameters; however, MDA was lower in the women aged 50–55 years compared with men, which may relate to a change of metabolism post-menopause [
5]. In the study by Weber et al., plasma 3-NOTyr correlated with subject age in the range of 55–75 years [
40]. These authors proposed the existence of different types of oxidative stress, some of which may depend on hormone levels.
For the markers in the urine, limited data are available, showing that similarly to our study, 8-OHdG in the urine was not gender-related [
3]. Interestingly, urine MDA levels were lower in the descendants from long-living families, theorized to be better genetically equipped to handle oxidative stress [
40]. In our study, these factors did not interfere with each other, as there were no women in the postmenopausal age and only one male above 65 years of age who participated in the control subject group in 2018.
Experimental data indicate that obesity is associated with increased oxidative stress, and data from human studies have shown that weight loss in obese patients leads to a decrease in markers of oxidative stress in white blood cells. These markers in urine still displayed a high variety of oxidized nucleic acids [
41]. In our study, the groups did not differ in BMI, and no correlation was observed.
The increased effect of smoking on the biomarkers has been found in several studies, as documented in a meta-analysis focusing on 8-OHdG and its increase in the urine [
3]. On the other hand, smoking did not affect 8-isoprostane levels in EBC [
1]. In our study, there was no difference in markers between groups of subjects for any bodily fluid studied, and the proportion of smokers was low. There was a minority of abstainers from alcohol in the groups studied. However, no consistent correlations between the levels of the markers in any fluid have been observed for smoking and alcohol intake.
A review of the studies by Brieger et al. has concluded that diet and drinking of non-alcoholic beverages may impact the concentration of oxidative stress markers and contribute to their variability [
42]. The effect may depend on the time interval of the sampling [
15]. For the volunteers, consumption of a median size meal (8.5 kcal/kg of body weight) increased 8-isoprostane levels in the plasma at 3 h and 6 h post-meal; however, levels did not increase in the EBC in the study by Kurti et al. [
43]. Drinking 1 L of Coke (pH 2.5) or mineral water (pH 6.5) decreased pH in the EBC, blood and urine of the volunteers within 15 min [
44].
In a controlled study by Kanabrocki et al. in 12 male, healthy volunteers, with a daily distribution of calories by a meal of 29% for breakfast, 30% for lunch and 41% for dinner, peak levels of urine 8-OHdG, MDA, and 8-isoprostane concentrations occurred in the evening, which may be attributable to the ingestion of a greater number of calories during dinner [
45]. In the workers and control subjects in this study, no difference in the concentration of these markers was observed if breakfast was consumed before the pre-shift/morning sampling, nor if their last meal was the previous evening’s dinner. Before and during EBC sampling, only tap water was provided for regular intake.
Habitual level of physical activity may impact 8-isoprostane production [
46], and its elevation in the EBC was found in a study by Kurti et al. after exhaustive exercise for postmenopausal women [
47]. The effect of intense work on MDA, another oxidative stress marker, is more complex. It may depend on the fitness of the subject and correlate inversely with the initial level of the given biomarker prior to exercise [
48].
In our previous study in 2016, dyspnea and chronic bronchitis in the exposed subjects were more frequent and correlated with pre-shift o-Tyr and post-shift 3-NOTyr levels in the EBC. In both years of follow-up, only one worker had a chronic cough, who had 18 years’ accumulated exposure to nanomaterials in 2017 and did not participate in 2018. Among the control subjects in 2018, one subject was accidentally included who complained of a cough first in the afternoon. This control subject had 3-NOTyr, MDA, 8-OHdG, and 5-OHMeU positively correlating with a cough, associated with newly appearing common cold symptoms. In the literature, the 3-NOTyr level was increased in patients with severe asthma, similarly to 8-isoprostane and further oxidative stress and inflammation EBC markers, such as leukotrienes C4–E4 [
49,
50].
In spirometry findings, a significant decrease in post-shift levels of % FEV1 and FEV1/FVC in the workers was observed in 2016. This was not observed in this follow-up study, which may be explained by the marginally shorter total length of exposure during the years 2017 and 2018.
Oxidative stress has been associated with aging, diabetes, cancer, neurodegenerative, among other disorders [
50]. In this study, the level of oxidative stress marker 8-OHG correlated with hypothyroidism treated with thyroxine. This disease was present in two female workers in 2017 and 2018, and in two female control subjects in 2018, as the only disease with a positive correlation with pre-shift 8-OHG plasma level in the workers. However, this correlation was not observed in the control subject samples in 2018. The relationship with oxidative stress is plausible; as for thyroid function, attaching an iodide to thyroglobulin requires hydrogen peroxide [
42]. However, no subject displayed typical hyper- or hypothyroidism symptoms.
A link between the molecular mechanisms of circadian rhythms and oxidative stress has been established, but it is still insufficiently understood. Importantly, oxidative stress biomarkers display considerable variation in healthy populations, and diurnal variability may vary for different biomarkers measured in different bodily fluids [
51]. For example, afternoon levels for plasma isoprostanes were lower in comparison with the morning samples [
52]. On the other hand, no significant circadian fluctuations of EBC, plasma and urinary MDA and 8-OHdG concentrations were seen in healthy young men at sampling times 8:30, 10:00, 11:00 and 13:00 [
30].
Urine samples, collected in healthy subjects daily in the morning over one month showed an intra-individual variability, which, from the set of seven markers, was the highest for 8-isoprostane and lowest for 8-OHdG levels, denoted as the most suitable biomarker for spot urine samples, normalized for creatinine concentration [
53]. Accordingly, a study by Barregard et al. found that the diurnal variability in 8-OHdG concentration for creatinine-adjusted results was a maximum of 6%, and no statistically significant impact of time was observed. However, the intraindividual variability between different days in 8-OHdG morning excretion was approximately 20% for creatinine-adjusted first void samples [
54]. Therefore, we cannot completely exclude the possibility of circadian effects in this study, which may influence the results for all subjects.
Importantly, we can exclude circadian variation as the main cause of elevation of oxidative stress markers in the workers handling nanoparticles. The main argument to support this conclusion is the fact that in our studies in nano-TiO
2 workers and nano-Fe-oxides workers, the collections of the pre-shift and/or post-shift samples were always carried out at different times of day and night over 24 h, as they worked in three 8 h shifts [
32,
33]. The circadian variation can, thus, be ruled out.
Environmental air contamination may cause inflammatory or immunological responses in the airways, which may alter EBC composition [
15]. In addition, markers in other bodily fluids may be affected, and the levels of MDA in the EBC and urine have been used as biomarkers of air pollution-induced oxidative stress [
55]. Such nanoparticles originating from iron-containing combustion and friction were detected in the nuclei of brain cells, with reduced DNA integrity in young deceased residents of highly air-polluted cities [
56]. In our previous studies, we did not confirm the effects of air pollution [
13,
14]. In this study, the environmental air pollution measured in the area was low, and no consistent positive correlation of these contaminants with oxidative stress markers was found.
6. Conclusions
It has been experimentally proven that oxidative stress due to nanomaterials exposure leads to inflammation, cell apoptosis and genotoxicity, and a chronic low-dose exposure also causes cellular reprogramming, disease initiation and other systemic damage [
12,
59]. Occupational medicine is facing a shortage of studies of biomarkers of effects related to exposure to nanomaterials for subjects with the highest exposure [
60,
61].
This study of nanocomposites in the handling workers found that both follow-up years, the pre-shift elevations of 50.0% in EBC, 37.5% in plasma and 6.25% in the urine biomarkers agreed with the previous results. Post-shift elevation reached 62.5% in EBC, 68.8% in plasma and 18.8% in urine samples. As to the post-shift acute elevation, it was seen in the workers despite the potential interference of other non-occupational factors discussed, as a significant post-shift decrease never occurred. The working procedure in which the subjects worked was not important since a high proportion of nanoparticles was measured during all procedures in all three years.
The conclusion of the first aim of this study is that the elevation of levels of the markers of oxidative stress in nanocomposite workers stays more or less stable over three years in the EBC and does not show trends, such as cumulative effect on the one side or adaptation on the other side. These results have been supported by follow-up analysis of the plasma and urine markers.
To obtain more significant results, a repeated collection of the post-shift samples after a longer time interval, such as 8–12 h, may be useful. In addition, individual data from the workers performing personal sampling of nanoparticles for measurement will be used in the future.
As to our second aim of the study, the answer is optimistic, as we believe that the sometimes-underestimated analysis of EBC samples proved to be a valuable method in comparison with other biological fluids studied. In addition, this approach showed identical trends for both plasma and urine, not influenced by the EBC concentration. This noninvasive method can be repeated without adverse events in short time intervals with good reproducibility [
62].
The effect of inhalation of nanoparticles is not limited to the respiratory tract, as the elevation of most plasma and urine markers occurred, and a systemic effect is plausible.
Concerning our third aim of the study, at minimum, the marker that should be included in the pre-shift markers in the EBC is 8-OHdG, as this marker was significantly elevated over all three following years. For the acute exposure, MDA appeared to be the most sensitive marker, as it elevated in the post-shift EBC every year. As stated, one single test is insufficient to assess oxidative stress, and optimally, the whole set of these eight biomarkers should be used, as they reportedly reflect different types of oxidative stress [
1,
2,
4,
5].
Clearly, other not fully known factors may also play a role. The methods for the analysis of the markers are highly sensitive, with fluctuations between the years, and it is hypothesized that they respond to other stimuli, such as genetics and lifestyle, as contributing factors to the individual status of oxidative stress. Therefore, a detailed assessment of individual parameters, including personal habits, diet, physical activity, time of sampling and environmental contamination data, should always be given.