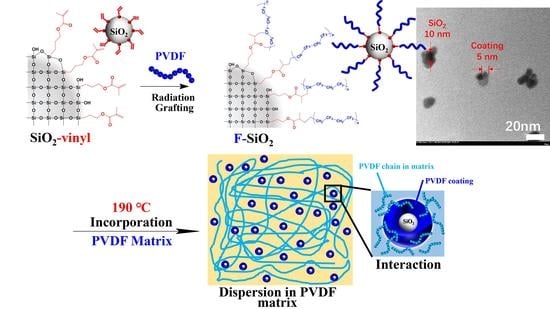
Radiation Induced Surface Modification of Nanoparticles and Their Dispersion in the Polymer Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedure for Exterior Functionalization of SiO2 with Vinyl Groups (SiO2-Vinyl)
2.3. General Procedure for Grafting PVDF Chains onto the Exterior Surface of SiO2-Vinyl by the “Radiation Grafting” Method (F-SiO2)
2.4. Preparation of Blending Materials
2.5. Characterizations
3. Results and Discussion
3.1. Preparation of F-SiO2: Functionalization of SiO2
3.2. Interface Modification Control of the PVDF Graft Ratio on Silica
3.3. Dispersion Property of Modification Silica in the PVDF Matrix
3.4. Modification Silica Loading Effect on the Physical Properties of the PVDF Matrix
3.4.1. Mechanical Properties
3.4.2. The Crystallization Behavior
3.4.3. The Thermomechanical Behavior
3.4.4. The Rheological Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pastore, V.J.; Cook, T.R. Coordination-Driven Self-Assembly in Polymer–Inorganic Hybrid Materials. Chem. Mater. 2020, 32, 3680–3700. [Google Scholar] [CrossRef]
- Dolbecq, A.; Dumas, E.; Mayer, C.R.; Mialane, P. Hybrid organic-inorganic polyoxometalate compounds: From structural diversity to applications. Chem. Rev. 2010, 110, 6009–6048. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of Hybrid Organic–Inorganic Nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.Y. Surfactant Behavior of Amphiphilic Polymer-Tethered Nanoparticles. Langmuir 2016, 32, 3567–3579. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, M.; Latorre-Sanchez, A.; Pomposo, J.A. Advances in single chain technology. Chem. Soc. Rev. 2015, 44, 6122–6142. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, Y.; Tian, J.; Zhao, H. Vesicles fabricated by hybrid nanoparticles. Chem. Commun. 2009, 25, 3807–3809. [Google Scholar] [CrossRef]
- Li, K.; Liang, S.; Lu, Y.; Wang, Q. Synthesis of Telechelic Fluoropolymers with Well-Defined Functional End Groups for Cross-Linked Networks and Nanocomposites. Macromolecules 2007, 40, 4121–4123. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Liang, X.; Tu, Z.; Zhang, H.; Feng, J.; Wan, Z.; Pan, M. Anhydrous Proton Conducting Materials Based on Sulfonated Dimethylphenethylchlorosilane Grafted Mesoporous Silica/Ionic Liquid Composite. ACS Appl. Mater. Interfaces 2013, 5, 11535–11543. [Google Scholar] [CrossRef]
- Tanahashi, M.; Hirose, M.; Watanabe, Y.; Lee, J.-C.; Takeda, K.J. Silica/Perfluoropolymer Nanocomposites Fabricated by Direct Melt-Compounding: A Novel Method without Surface Modification on Nano-Silica. J. Nanosci. Nanotechnol. 2007, 7, 1–10. [Google Scholar] [CrossRef]
- Darr, J.A.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous hydrothermal synthesis of inorganic nanoparticles: Applications and future directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef] [Green Version]
- Gravano, S.M.; Dumas, R.; Liu, K.; Patten, T.E. Methods for the surface functionalization of γ-Fe2O3 nanoparticles with initiators for atom transfer radical polymerization and the formation of core–shell inorganic–polymer structures. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3675–3688. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Yuan, L.; Yang, Y.; Liu, L.; Zhao, H. Self-assembly of monotethered single-chain nanoparticle shape amphiphiles. ACS Macro Lett. 2013, 2, 100–106. [Google Scholar] [CrossRef]
- Kayser, M.J.; Reinholdt, M.X.; Kaliaguine, S. Amine grafted silica/SPEEK nanocomposites as proton exchange membranes. J. Phys. Chem. B 2010, 114, 8387–8395. [Google Scholar] [CrossRef]
- Liao, Z.; Wu, G.; Lee, D.; Yang, S. Ultrastable Underwater Anti-Oil Fouling Coatings from Spray Assemblies of Polyelectrolyte Grafted Silica Nanochains. ACS Appl. Mater. Interfaces 2019, 11, 13642–13651. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zhu, W.; Cui, Y. Synthesis of Polymeric Nanocomposite Hydrogels Containing the Pendant ZnS Nanoparticles: Approach to Higher Refractive Index Optical Polymeric Nanocomposites. Macromolecules 2018, 51, 2672–2681. [Google Scholar] [CrossRef]
- Durand, N.; Boutevin, B.; Silly, G.; Ameduri, B. “Grafting From” Polymerization of Vinylidene Fluoride (VDF) from Silica to Achieve Original Silica–PVDF Core–Shells. Macromolecules 2011, 44, 8487–8493. [Google Scholar] [CrossRef]
- Durand, N.; Gaveau, P.; Silly, G.; Ameduri, B.; Boutevin, B. Radical Grafting of Tetrafluoroethylene and Vinylidene Fluoride Telomers onto Silica Bearing Vinyl Groups. Macromolecules 2011, 44, 6249–6257. [Google Scholar] [CrossRef]
- Pribyl, J.; Benicewicz, B.; Bell, M.; Wagener, K.; Ning, X.; Schadler, L.; Jimenez, A.; Kumar, S. Polyethylene Grafted Silica Nanoparticles Prepared via Surface-Initiated ROMP. ACS Macro Lett. 2019, 8, 228–232. [Google Scholar] [CrossRef]
- Vukicevic, R.; Beuermann, S. Fullerenes Decorated with Poly (vinylidene fluoride). Macromolecules 2011, 44, 2597–2603. [Google Scholar] [CrossRef]
- Nasef, M.M. Radiation-grafted membranes for polymer electrolyte fuel cells: Current trends and future directions. Chem. Rev. 2014, 114, 12278–12329. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F. High-energy radiation induced sustainable coloration and functional finishing of textile materials. Ind. Eng. Chem. Res. 2015, 54, 3727–3745. [Google Scholar]
- Nasef, M.M.; Hegazy, E.S.A. Preparation and applications of ion exchange membranes by radiation-induced graft copolymerization of polar monomers onto non-polar films. Prog. Polym. Sci. 2004, 29, 499–561. [Google Scholar] [CrossRef]
- Dargaville, T.R.; George, G.A.; Hill, D.J.; Whittaker, A.K. High energy radiation grafting of fluoropolymers. Prog. Polym. Sci. 2003, 28, 1355–1376. [Google Scholar] [CrossRef]
- Hu, J.; Gao, Q.; Xu, L.; Zhang, M.; Xing, Z.; Guo, X.; Zhang, K.; Wu, G. Significant improvement in thermal and UV resistances of UHMWPE fabric through in situ formation of polysiloxane–TiO2 hybrid layers. ACS Appl. Mater. Interfaces 2016, 8, 23311–23320. [Google Scholar] [CrossRef]
- Wang, H.; Fu, Z.; Zhao, X.; Li, Y.; Li, J. Reactive Nanoparticles Compatibilized Immiscible Polymer Blends: Synthesis of Reactive SiO2 with Long Poly(methyl methacrylate) Chains and the in Situ Formation of Janus SiO2 Nanoparticles Anchored Exclusively at the Interface. ACS Appl. Mater. Interfaces 2017, 9, 14358–14370. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Fu, Z.; Li, Y. Enhanced Interfacial Adhesion by Reactive Carbon Nanotubes: New Route to High-Performance Immiscible Polymer Blend Nanocomposites with Simultaneously Enhanced Toughness, Tensile Strength, and Electrical Conductivity. ACS Appl. Mater. Interfaces 2018, 10, 8411–8416. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, H.; Zhao, X.; Li, X.; Gu, X.; Li, Y. Flame-retarding nanoparticles as the compatibilizers for immiscible polymer blends: Simultaneously enhanced mechanical performance and flame retardancy. J. Mater. Chem. A 2019, 7, 4903–4912. [Google Scholar] [CrossRef]
- Constantin, L.V.; Iconaru, S.; Ciobanu, C.S. Europium doped hydroxyapatite for applications in environmental field. Rom. Rep. Phys. 2012, 64, 788–794. [Google Scholar]
- Ciobanu, C.S.; Andronescu, E.; Prodan, A.M.; Pall, L.; Iconaru, S.L. Physico-chemical and antibacterial studies on silver doped nano-hydroxyapatite. J. Optoelectron. Adv. Mater. 2013, 15, 918–922. [Google Scholar]
- Iconaru, S.L.; Prodan, A.M.; Turculet, C.S.; Beuran, M.; Ghita, R.V.; Costescu, A.; Groza, A.; Chifiriuc, M.C.; Chapon, P.; Gaiaschi, S.; et al. Enamel based composite layers deposited on titanium substrate with antifungal activity. J. Spectrosc. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Ghita, R.; Iconaru, S.L.; Popa, C.; Costescu, A.; Coustumer, P.L.; Motelicaheino, M.; Ciobanu, C.S. Tetraethyl orthosilicate coated hydroxyapatite powders for lead ions removal from aqueous solutions. J. Nanomater. 2014, 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Iconaru, S.L.; Turculet, C.; Coustumer, P.L.; Bleotu, C.; Prodan, A.M. Biological studies on dextrin coated iron oxide nanoparticles. Rom. Rep. Phys. 2016, 68, 1536–1544. [Google Scholar]
- Li, H.; Yan, S. Surface-induced polymer crystallization and the resultant structures and morphologies. Macromolecules 2011, 44, 417–428. [Google Scholar] [CrossRef]
- Lotz, B.; Miyoshi, T.; Cheng, S.Z. 50th anniversary perspective: Polymer crystals and crystallization: Personal journeys in a challenging research field. Macromolecules 2017, 50, 5995–6025. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Y.; Sarkar, A.; Xu, Y.; Stefik, M.; Benicewicz, B.C. Matrix-Free Polymer Nanocomposite Thermoplastic Elastomers. Macromolecules 2017, 50, 4742–4753. [Google Scholar] [CrossRef]
- Frantisek, O.; Petr, L.; Marek, Z.; Klara, Z.; Leon, E.G.; Josef, J. Effect of Nanoparticle Organization on Molecular Mobility and Mechanical Properties of Polymer Nanocomposites. Macromolecules 2019, 52, 6250–6259. [Google Scholar]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Gu, X.; Hu, L.; Li, Y.; Li, J. Radiation Induced Surface Modification of Nanoparticles and Their Dispersion in the Polymer Matrix. Nanomaterials 2020, 10, 2237. https://doi.org/10.3390/nano10112237
Fu Z, Gu X, Hu L, Li Y, Li J. Radiation Induced Surface Modification of Nanoparticles and Their Dispersion in the Polymer Matrix. Nanomaterials. 2020; 10(11):2237. https://doi.org/10.3390/nano10112237
Chicago/Turabian StyleFu, Zhiang, Xiaoying Gu, Lingmin Hu, Yongjin Li, and Jingye Li. 2020. "Radiation Induced Surface Modification of Nanoparticles and Their Dispersion in the Polymer Matrix" Nanomaterials 10, no. 11: 2237. https://doi.org/10.3390/nano10112237