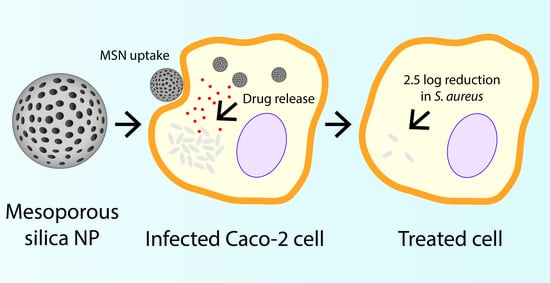
Enhancing the Cellular Uptake and Antibacterial Activity of Rifampicin through Encapsulation in Mesoporous Silica Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Mesoporous Silica Nanoparticles (MSN)
2.1.1. Synthesis of MSN
2.1.2. Rhodamine B Loading into MSN
2.1.3. Rifampicin Loading into MSN
2.2. Physicochemical Characterization of MSN
2.2.1. Particle Sizing and Structure
2.2.2. Nitrogen Adsorption/Desorption Isotherms
2.2.3. Thermogravimetric Analysis (TGA)
2.2.4. Fourier Transform Infra-Red Attenuated Total Reflection (FTIR-ATR) Spectroscopy
2.3. Quartz Crystal Microbalance with Dissipation (QCM-D) Studies
2.4. Total Internal Reflection Fluorescence (TIRF) Microscopy Studies
2.4.1. Preparation of Semi-Native Lipid Vesicles
2.4.2. Preparation of Fluorescent Tracer Vesicles
2.4.3. Formation of a Semi-Native Supported Lipid Bilayer (snSLB)
2.4.4. Monitoring of MSN Adsorption onto snSLB
2.5. In Vitro Cellular Uptake Studies
2.5.1. Cellular Uptake of MSN Particles with Flow Cytometry
2.5.2. Cellular Uptake of MSN Particles with Confocal Microscopy
2.6. In Vitro Cell Viability Studies
2.7. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of MSN
3.2. Cell Viability and Caco-2 Uptake of MSN
3.3. Biophysical Analysis of MSN Adsorption onto Biologically Relevant Lipid Bilayers
3.4. Antibacterial Efficacy of Rifampicin-Loaded MSN against Intracellular Pathogens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garzoni, C.; Kelley, W.L. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009, 17, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sendi, P.; Proctor, R.A. Staphylococcus aureus as an intracellular pathogen: The role of small colony variants. Trends Microbiol. 2009, 17, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Khuller, G. Antitubercular inhaled therapy: Opportunities, progress and challenges. J. Antimicrob. Chemother. 2005, 55, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Mackanes, G. Resistance to intracellular infection. J. Infect. Dis. 1971, 123, 439–445. [Google Scholar] [CrossRef]
- Gonçalves, J.E.; Ballerini Fernandes, M.; Chiann, C.; Gai, M.N.; De Souza, J.; Storpirtis, S. Effect of pH, mucin and bovine serum on rifampicin permeability through Caco-2 cells. Biopharm. Drug Dispos. 2012, 33, 316–323. [Google Scholar] [CrossRef]
- Tulkens, P.M. Intracellular distribution and activity of antibiotics. Eur. J. Clin. Microbiol. Infecti. Dis. 1991, 10, 100–106. [Google Scholar] [CrossRef]
- Mounier, J.; Ryter, A.; Coquis-Rondon, M.; Sansonetti, P. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect. Immun. 1990, 58, 1048–1058. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Cooper, A.; Sturgill-Koszycki, S.; Van Heyningen, T.; Chatterjee, D.; Orme, I.; Allen, P.; Russell, D.G. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 1994, 153, 2568–2578. [Google Scholar]
- Bennett, C.L.; Misslitz, A.; Colledge, L.; Aebischer, T.; Blackburn, C.C. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. Eur. J. Immunol. 2001, 31, 876–883. [Google Scholar] [CrossRef]
- Maghrebi, S.; Prestidge, C.A.; Joyce, P. An update on polymer-lipid hybrid systems for improving oral drug delivery. Expert Opin. Drug Deliv. 2019, 16, 507–524. [Google Scholar] [CrossRef]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.F.; Abdelkhalek, A.; Seleem, M.N. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Flórez, J.; Torres, R.; Urquiza, M.; Gutiérrez, J.; Guzmán, F.; Ortiz, C. Antimicrobial activity of a new synthetic peptide loaded in polylactic acid or poly (lactic-co-glycolic) acid nanoparticles against Pseudomonas aeruginosa, Escherichia coli O157: H7 and methicillin resistant Staphylococcus aureus (MRSA). Nanotechnology 2017, 28, 135102. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Thomas, N.; Gustafsson, H.; Jambhrunkar, M.; Kidd, P.S.; Prestidge, A.C. Rifampicin-Loaded Mesoporous Silica Nanoparticles for the Treatment of Intracellular Infections. Antibiotics 2019, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasir, J.K.; Reddy, M.K.; Labhasetwar, V.D. Nanosystems in drug targeting: Opportunities and challenges. Curr. Nanosci. 2005, 1, 47–64. [Google Scholar] [CrossRef]
- Maghrebi, S.; Joyce, P.; Jambhrunkar, M.; Thomas, N.; Prestidge, C.A. PLGA-Lipid Hybrid (PLH) Microparticles Enhance the Intracellular Uptake and Antibacterial Activity of Rifampicin. ACS Appl. Mater. Interfaces 2020, 12, 8030–8039. [Google Scholar] [CrossRef] [PubMed]
- Santovito, E.; das Neves, J.; Greco, D.; D’Ascanio, V.; Sarmento, B.; Logrieco, A.F.; Avantaggiato, G. Antimicrobial properties of rosin acids-loaded nanoparticles against antibiotic-sensitive and antibiotic-resistant foodborne pathogens. Artif. Cells Nanomed. Biotechnol. 2018, 46, S414–S422. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Diab, R.; Joubert, O.; Canilho, N.; Pasc, A. Core–shell microcapsules of solid lipid nanoparticles and mesoporous silica for enhanced oral delivery of curcumin. Colloids Surf. B Biointerfaces 2016, 140, 161–168. [Google Scholar] [CrossRef]
- Zheng, N.; Li, J.; Xu, C.; Xu, L.; Li, S.; Xu, L. Mesoporous silica nanorods for improved oral drug absorption. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Pocock, K.; Delon, L.C.; Khatri, A.; Prestidge, C.; Gibson, R.; Barbe, C.; Thierry, B. Uptake of silica particulate drug carriers in an intestine-on-a-chip: Towards a better in vitro model of nanoparticulate carrier and mucus interactions. Biomater. Sci. 2019, 7, 2410–2420. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Cui, Y.; Zhao, Q.; Zhang, Q.; Musetti, S.; Kinghorn, K.A.; Wang, S. Overcoming multiple gastrointestinal barriers by bilayer modified hollow mesoporous silica nanocarriers. Acta Biomater. 2018, 65, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Qiao, S.Z.; Lu, G.Q.M. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.-H.; Hung, Y.; Mou, C.-Y. Mesoporous silica nanoparticles as nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.T.; Popat, A.; Mahony, D.; Cavallaro, A.S.; Yu, C.; Mitter, N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale 2013, 5, 5167–5179. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Li, C.; Qin, Y.; Yin, R.; Li, X.; Tian, M.; Du, S.; Guo, H.; Liu, C.; Zhu, N.; et al. Inhibition of Staphylococcus aureus adherence to Caco-2 cells by lactobacilli and cell surface properties that influence attachment. Anaerobe 2012, 18, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Kim, S.-G.; Iskandar, F.; Okuyama, K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous Mesoporous Mater. 2009, 120, 447–453. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhi, Z.; Jiang, T.; Zhang, J.; Wang, Z.; Wang, S. Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J. Control. Release 2010, 145, 257–263. [Google Scholar] [CrossRef]
- Gustafsson, H.; Isaksson, S.; Altskär, A.; Holmberg, K. Mesoporous silica nanoparticles with controllable morphology prepared from oil-in-water emulsions. J. Colloid Interface Sci. 2016, 467, 253–260. [Google Scholar] [CrossRef]
- Pace, H.; Simonsson Nyström, L.; Gunnarsson, A.; Eck, E.; Monson, C.; Geschwindner, S.; Snijder, A.; Höök, F. Preserved transmembrane protein mobility in polymer-supported lipid bilayers derived from cell membranes. Anal. Chem. 2015, 87, 9194–9203. [Google Scholar] [CrossRef]
- Pace, H.P.; Hannestad, J.K.; Armonious, A.; Adamo, M.; Agnarsson, B.; Gunnarsson, A.; Micciulla, S.; Sjövall, P.; Gerelli, Y.; Höök, F. Structure and composition of native membrane derived polymer-supported lipid bilayers. Anal. Chem. 2018, 90, 13065–13072. [Google Scholar] [CrossRef]
- Jönsson, P.; Jonsson, M.P.; Tegenfeldt, J.O.; Höök, F. A method improving the accuracy of fluorescence recovery after photobleaching analysis. Biophys. J. 2008, 95, 5334–5348. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Clemens, D.L.; Lee, B.-Y.; Xue, M.; Thomas, C.R.; Meng, H.; Ferris, D.; Nel, A.E.; Zink, J.I.; Horwitz, M.A. Targeted intracellular delivery of antituberculosis drugs to Mycobacterium tuberculosis-infected macrophages via functionalized mesoporous silica nanoparticles. Antimicrob. Agents Chemother. 2012, 56, 2535–2545. [Google Scholar] [CrossRef] [Green Version]
- Cortesi, R.; Esposito, E.; Menegatti, E.; Gambari, R.; Nastruzzi, C. Effect of cationic liposome composition on in vitro cytotoxicity and protective effect on carried DNA. Int. J. Pharm. 1996, 139, 69–78. [Google Scholar] [CrossRef]
- Schachter, D. The Source of Toxicity in CTAB and CTAB-Stabilized Gold Nanorods; Rutgers University-Graduate School: New Brunswick, NJ, USA, 2013. [Google Scholar]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, Y.J.; Zhu, L.; Teo, W.S.; Tan, Y.W.; Yang, Y.; Wang, C.; Chen, H. Revisiting the Stöber Method: Inhomogeneity in Silica Shells. J. Am. Chem. Soc. 2011, 133, 11422–11425. [Google Scholar] [CrossRef] [PubMed]
- Khraisheh, M.; Al-Ghouti, M.; Allen, S.; Ahmad, M. Effect of OH and silanol groups in the removal of dyes from aqueous solution using diatomite. Water Res. 2005, 39, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Asefa, T.; MacLachlan, M.J.; Coombs, N.; Ozin, G.A. Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 1999, 402, 867–871. [Google Scholar] [CrossRef]
- Hua, Z.-L.; Shi, J.-L.; Wang, L.; Zhang, W.-H. Preparation of mesoporous silica films on a glass slide: Surfactant template removal by solvent extraction. J. Non-Cryst. Solids 2001, 292, 177–183. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.G.; Airoldi, C. Different neutral surfactant template extraction routes for synthetic hexagonal mesoporous silicas. J. Mater. Chem. 2002, 12, 3823–3826. [Google Scholar] [CrossRef] [Green Version]
- van de Loosdrecht, A.A.; Nennie, E.; Ossenkoppele, G.J.; Beelen, R.H.; Langenhuijsen, M.M. Cell mediated cytotoxicity against U 937 cells by human monocytes and macrophages in a modified colorimetric MTT assay: A methodological study. J. Immunol. Methods 1991, 141, 15–22. [Google Scholar] [CrossRef]
- Nash, T.; Allison, A.; Harington, J. Physico-chemical properties of silica in relation to its toxicity. Nature 1966, 210, 259–261. [Google Scholar] [CrossRef]
- Slowing, I.I.; Wu, C.-W.; Vivero-Escoto, J.L.; Lin, V.S.Y. Mesoporous Silica Nanoparticles for Reducing Hemolytic Activity Towards Mammalian Red Blood Cells. Small 2009, 5, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Haynes, C.L. Impacts of Mesoporous Silica Nanoparticle Size, Pore Ordering, and Pore Integrity on Hemolytic Activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef]
- Sun, B.; Pokhrel, S.; Dunphy, D.R.; Zhang, H.; Ji, Z.; Wang, X.; Wang, M.; Liao, Y.-P.; Chang, C.H.; Dong, J. Reduction of acute inflammatory effects of fumed silica nanoparticles in the lung by adjusting silanol display through calcination and metal doping. ACS Nano 2015, 9, 9357–9372. [Google Scholar] [CrossRef] [Green Version]
- Faria, M.; Björnmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond) 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Bu, L.; Xie, J.; Chen, K.; Cheng, Z.; Li, X.; Chen, X. Effects of Nanoparticle Size on Cellular Uptake and Liver MRI with Polyvinylpyrrolidone-Coated Iron Oxide Nanoparticles. ACS Nano 2010, 4, 7151–7160. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Huang, N.; Li, H.; Jin, Q.; Ji, J. Surface and Size Effects on Cell Interaction of Gold Nanoparticles with Both Phagocytic and Nonphagocytic Cells. Langmuir 2013, 29, 9138–9148. [Google Scholar] [CrossRef]
- Lu, F.; Wu, S.-H.; Hung, Y.; Mou, C.-Y. Size Effect on Cell Uptake in Well-Suspended, Uniform Mesoporous Silica Nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Pyrgiotakis, G.; Beltran-Huarac, J.; Zhang, Y.; Gaurav, J.; Deloid, G.; Spyrogianni, A.; Sarosiek, K.A.; Bello, D.; Demokritou, P. Safer-by-design flame-sprayed silicon dioxide nanoparticles: The role of silanol content on ROS generation, surface activity and cytotoxicity. Part. Fibre Toxicol. 2019, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Peerboom, N.; Schmidt, E.; Trybala, E.; Block, S.; Bergström, T.; Pace, H.P.; Bally, M. Cell Membrane Derived Platform To Study Virus Binding Kinetics and Diffusion with Single Particle Sensitivity. ACS Infect. Dis. 2018, 4, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.L.; Burghardt, T.P.; Axelrod, D. Measuring surface dynamics of biomolecules by total internal reflection fluorescence with photobleaching recovery or correlation spectroscopy. Biophys. J. 1981, 33, 435–454. [Google Scholar] [CrossRef] [Green Version]
- Axelrod, D.; Thompson, N.L.; Burghardt, T.P. Total internal reflection fluorescent microscopy. J. Microsc. 1983, 129, 19–28. [Google Scholar] [CrossRef]
- Larsen, J.; Hatzakis, N.S.; Stamou, D. Observation of inhomogeneity in the lipid composition of individual nanoscale liposomes. J. Am. Chem. Soc. 2011, 133, 10685–10687. [Google Scholar] [CrossRef]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Oh, W.-K.; Kim, S.; Choi, M.; Kim, C.; Jeong, Y.S.; Cho, B.-R.; Hahn, J.-S.; Jang, J. Cellular uptake, cytotoxicity, and innate immune response of silica− titania hollow nanoparticles based on size and surface functionality. ACS Nano 2010, 4, 5301–5313. [Google Scholar] [CrossRef]
- Ekkapongpisit, M.; Giovia, A.; Follo, C.; Caputo, G.; Isidoro, C. Biocompatibility, endocytosis, and intracellular trafficking of mesoporous silica and polystyrene nanoparticles in ovarian cancer cells: Effects of size and surface charge groups. Int. J. Nanomed. 2012, 7, 4147. [Google Scholar]
- Hess, D.J.; Henry-Stanley, M.J.; Erickson, E.; Wells, C.L. Intracellular survival of Staphylococcus aureus within cultured enterocytes. J. Surg. Res. 2003, 114, 42–49. [Google Scholar] [CrossRef]
- Kwak, Y.-K.; Vikström, E.; Magnusson, K.-E.; Vécsey-Semjén, B.; Colque-Navarro, P.; Möllby, R. The Staphylococcus aureus alpha-toxin perturbs the barrier function in Caco-2 epithelial cell monolayers by altering junctional integrity. Infect. Immun. 2012, 80, 1670–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, M.B.; Gonçalves, J.E.; Scotti, M.T.; de Oliveira, A.A.; Tavares, L.C.; Storpirtis, S. Caco-2 cells cytotoxicity of nifuroxazide derivatives with potential activity against Methicillin-resistant Staphylococcus aureus (MRSA). Toxicol. In Vitro 2012, 26, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A.; Peters, G. Small colony variants in staphylococcal infections: Diagnostic and therapeutic implications. Clin. Infect. Dis. 1998, 27, 419–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, R.A.; Von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef]
- Joyce, P.; Ulmefors, H.; Garcia-Bennett, A.E.; Prestidge, C.A. Microporosity, Pore Size, and Diffusional Path Length Modulate Lipolysis Kinetics of Triglycerides Adsorbed onto SBA-15 Mesoporous Silica Particles. Langmuir 2020, in press. [Google Scholar] [CrossRef]




| Particle | Surfactant Extraction Protocol | Mean Particle Size (nm) | Mean Pore Width (nm) | Specific Pore Volume (cm3/g) | Specific Surface Area (m2/g) | Zeta Potential (mV) | Drug Loading (% w/w) |
|---|---|---|---|---|---|---|---|
| MSN40e | Solvent extraction | 47.0 ± 7.0 | 11.7 | 1.30 | 483 | −15.1 ± 5.4 | 28.9 |
| MSN40c | Calcination | 47.0 ± 7.0 | 11.7 | 1.30 | 483 | −13.6 ± 6.3 | 33.6 |
| MSN80c | Calcination | 84.1 ± 17.4 | 7.46 | 0.81 | 460 | −14.9 ± 4.7 | 38.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joyce, P.; Ulmefors, H.; Maghrebi, S.; Subramaniam, S.; Wignall, A.; Jõemetsa, S.; Höök, F.; Prestidge, C.A. Enhancing the Cellular Uptake and Antibacterial Activity of Rifampicin through Encapsulation in Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 815. https://doi.org/10.3390/nano10040815
Joyce P, Ulmefors H, Maghrebi S, Subramaniam S, Wignall A, Jõemetsa S, Höök F, Prestidge CA. Enhancing the Cellular Uptake and Antibacterial Activity of Rifampicin through Encapsulation in Mesoporous Silica Nanoparticles. Nanomaterials. 2020; 10(4):815. https://doi.org/10.3390/nano10040815
Chicago/Turabian StyleJoyce, Paul, Hanna Ulmefors, Sajedeh Maghrebi, Santhni Subramaniam, Anthony Wignall, Silver Jõemetsa, Fredrik Höök, and Clive A. Prestidge. 2020. "Enhancing the Cellular Uptake and Antibacterial Activity of Rifampicin through Encapsulation in Mesoporous Silica Nanoparticles" Nanomaterials 10, no. 4: 815. https://doi.org/10.3390/nano10040815