DFT Studies of the Activity and Reactivity of Limonene in Comparison with Selected Monoterpenes
Abstract
:1. Introduction
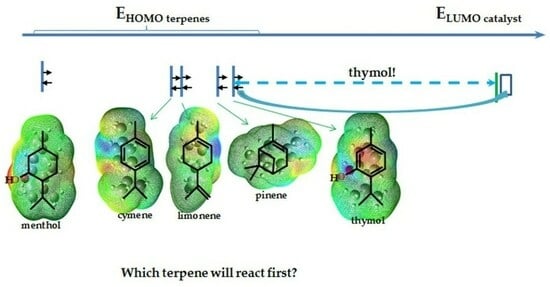
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandelli, D.; Kozlov, Y.N.; da Silva, C.A.R.; Carvalho, W.A.; Pescarmona, P.P.; Cella, D.d.A.; de Paiva, P.T.; Shul’pin, G.B. Oxidation of olefins with H2O2 catalyzed by gallium(III) nitrate and aluminum(III) nitrate in solution. J. Mol. Catal. A Chem. 2016, 422, 216–220. [Google Scholar] [CrossRef]
- Santos, I.C.M.S.; Gamelas, J.A.F.; Duarte, T.A.G.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Cavaleiro, A.M.V. Catalytic homogeneous oxidation of monoterpenes and cyclooctene with hydrogen peroxide in the presence of sandwich-type tungstophosphates [M4(H2O)2(PW9O34)2]n−, M = CoII, MnII and FeIII. J. Mol. Catal. A Chem. 2017, 426, 593–599. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Al-Jaloudi, R.; Abdullah, M.A.; Abughoush, M. Characterization of Olive Oil Volatile Compounds after Elution through Selected Bleaching Materials-Gas Chromatography-Mass Spectrometry Analysis. Molecules 2023, 28, 6444. [Google Scholar] [CrossRef] [PubMed]
- Wikandari, R.; Nguyen, H.; Millati, R.; Niklasson, C.; Taherzadeh, M.J. Improvement of Biogas Production from Orange Peel Waste by Leaching of Limonene. BioMed Res. Int. 2015, 2015, 494182. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska-Mizera, M.; Knitter, M.; Szymanowska, D.; Mallardo, S.; Santagata, G.; Di Lorenzo, M.L. Optical, mechanical, and antimicrobial properties of bio-based composites of poly(L-lactic acid) and D-limonene/β-cyclodextrin inclusion complex. J. App. Polym. Sci. 2022, 139, 52177. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Knitter, M.; Piss, M.; Szymanowska, D.; Mallardo, S.; Santagata, G.; Di Lorenzo, M.L. Linear low-density polyethylene modified with d-limonene/β-cyclodextrin inclusion complex: Antimicrobial composite for active food packaging. Polym. Eng. Sci. 2024, 64, 52–61. [Google Scholar] [CrossRef]
- Saldanha do Carmo, C.; Pais, R.; Simplício, A.L.; Mateus, M.; Duarte, C.M.M. Improvement of Aroma and Shelf-Life of Non-alcoholic Beverages Through Cyclodextrins-Limonene Inclusion Complexes. Food Bioprocess Technol. 2017, 10, 1297–1309. [Google Scholar] [CrossRef]
- Davaritouchaee, M.; Mosleh, I.; Dadmohammadi, Y.; Abbaspourrad, A. One-Step Oxidation of Orange Peel Waste to Carbon Feedstock for Bacterial Production of Polyhydroxybutyrate. Polymers 2023, 15, 697. [Google Scholar] [CrossRef]
- Giang, P.D.; Churchman, L.R.; Stok, J.E.; Bell, S.G.; De Voss, J.J. Cymredoxin, a [2Fe-2S] ferredoxin, supports catalytic activity of the p-cymene oxidising P450 enzyme CYP108N12. Arch. Biochem. Biophys. 2023, 737, 109549. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Ji, G.; Li, A. Refinement of limonene epoxides from the light distillate of tire pyrolysis oil via catalytic epoxidation. Sep. Purif. Technol. 2023, 319, 124068. [Google Scholar] [CrossRef]
- Makrygenni, O.; Vanmairis, L.; Taourit, S.; Launay, F.; Shum Cheong Sing, A.; Proust, A.; Gérard, H.; Villanneau, R. Selective Formation of Epoxylimonene Catalyzed by Phosphonyl/Arsonyl Derivatives of Trivacant Polyoxotungstates at Low Temperature. Eur. J. Inorg. Chem. 2020, 2020, 605–612. [Google Scholar] [CrossRef]
- Kern, S.; Granier, T.; Dkhil, H.; Haupt, T.; Ellis, G.; Natsch, A. Stability of limonene and monitoring of a hydroperoxide in fragranced products. Flavour Fragr. J. 2014, 29, 277–286. [Google Scholar] [CrossRef]
- Christensson, J.B.; Johansson, S.; Hagvall, L.; Jonsson, C.; Börje, A.; Karlberg, A.T. Limonene hydroperoxide analogues differ in allergenic activity. Contact Dermat. 2008, 59, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Kaade, W.; Méndez-Sánchez, C.; Güell, C.; De Lamo-Castellví, S.; Mestres, M.; Ferrando, M. Complexed Biopolymer of Whey Protein and Carboxymethyl Cellulose to Enhance the Chemical Stability of Lemon Oil-in-Water Emulsions. ACS Food Sci. Technol. 2022, 2, 41–48. [Google Scholar] [CrossRef]
- Francisco-Márquez, M.; Galano, A. Limonene: A scented and versatile tropospheric free radical deactivator. Int. J. Quantum Chem. 2023, 123, e27103. [Google Scholar] [CrossRef]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 120, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Liang, X. Electrospun Polyvinyl Alcohol/d-Limonene Fibers Prepared by Ultrasonic Processing for Antibacterial Active Packaging Material. Molecules 2019, 24, 767. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Pant, A.; Pandey, R.K. Limonene Attenuates Oxidative Stress and Extends Longevity in Caenorhabditis elegans. Curr. Sci. 2019, 116, 959–965. [Google Scholar] [CrossRef]
- Sales, A.; Pastore, G.M.; Bicas, J.L. Optimization of limonene biotransformation to limonene-1,2-diol by Colletotrichum nymphaeae CBMAI 0864. Process Biochem. 2019, 86, 25–31. [Google Scholar] [CrossRef]
- De Oliveira, M.P.; Delolo, F.G.; Villarreal, J.A.A.; dos Santos, E.N.; Gusevskaya, E.V. Hydroformylation and one-pot hydroformylation/epoxy ring cleavage of limonene oxide: A sustainable access to biomass-based multi-functional fragrances. Appl. Catal. A Gen. 2021, 616, 118082. [Google Scholar] [CrossRef]
- Aissou, M.; Chemat-Djenni, Z.; Yara-Varón, E.; Fabiano-Tixier, A.-S.; Chemat, F. Limonene as an agro-chemical building block for the synthesis and extraction of bioactive compounds. Comptes Rendus Chim. 2017, 20, 346–358. [Google Scholar] [CrossRef]
- Claudino, M.; Mathevet, J.-M.; Jonsson, M.; Johansson, M. Bringing d-limonene to the scene of bio-based thermoset coatings via free-radical thiol–ene chemistry: Macromonomer synthesis, UV-curing and thermo-mechanical characterization. Polym. Chem. 2014, 5, 3245–3260. [Google Scholar] [CrossRef]
- Hauenstein, O.; Reiter, M.; Agarwal, S.; Rieger, B.; Greiner, A. Bio-based polycarbonate from limonene oxide and CO2 with high molecular weight, excellent thermal resistance, hardness and transparency. Green Chem. 2016, 18, 760–770. [Google Scholar] [CrossRef]
- Li, C.; Sablong, R.J.; van Benthem, R.A.T.M.; Koning, C.E. Unique Base-Initiated Depolymerization of Limonene-Derived Polycarbonates. ACS Macro Lett. 2017, 6, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.C.; Silvino, C.A. A Brief Review of the Use of Limonene Derivative as a Promising Monomer in the Synthesis of Biodegradable Polymers. Rev. Virtual Química 2021, 13, 1017–1041. [Google Scholar] [CrossRef]
- Nunes, M.S.; Gomes, D.M.; Gomes, A.C.; Neves, P.; Mendes, R.F.; Paz, F.A.A.; Lopes, A.D.; Valente, A.A.; Gonçalves, I.S.; Pillinger, M. A 5-(2-Pyridyl)tetrazolate Complex of Molybdenum(VI), Its Structure, and Transformation to a Molybdenum Oxide-Based Hybrid Heterogeneous Catalyst for the Epoxidation of Olefins. Catalysts 2021, 11, 1407. [Google Scholar] [CrossRef]
- Kuznetsova, G.M.; Lobanova, T.V.; Rusina, I.F.; Kasaikina, O.T. Kinetic characteristics of initiated oxidation of limonene. Russ. Chem. Bull. 1996, 45, 1586–1591. [Google Scholar] [CrossRef]
- Wentzel, B.B.; Alsters, P.L.; Feiters, M.C.; Nolte, R.J.M. Mechanistic Studies on the Mukaiyama Epoxidation. J. Org. Chem. 2004, 69, 3453–3464. [Google Scholar] [CrossRef]
- Pena, A.; Veiga, S.; Sapelli, M.; Martínez, N.; Márquez, V.; Dellacassa, E.; Bussi, J. Limonene oxidation by molecular oxygen under solvent-free conditions: The influence of peroxides and catalysts on the reaction rate. React. Kinet. Mech. Catal. 2012, 107, 263–275. [Google Scholar] [CrossRef]
- Martinez, Q.H.; Amaya, Á.A.; Paez-Mozo, E.A.; Martinez, O.F.; Valange, S. Photo-assisted O-atom transfer to monoterpenes with molecular oxygen and a dioxoMo(VI) complex immobilized on TiO2 nanotubes. Catal. Today 2021, 375, 441–457. [Google Scholar] [CrossRef]
- Martínez, Q.H.; Paez-Mozo, E.A.; Martínez, O.F. Selective Photo-epoxidation of (R)-(+)- and (S)-(−)-Limonene by Chiral and Non-Chiral Dioxo-Mo(VI) Complexes Anchored on TiO2-Nanotubes. Top. Catal. 2021, 64, 36–50. [Google Scholar] [CrossRef]
- Martinez Quiñonez, H.; Amaya, Á.A.; Paez-Mozo, E.A.; Martinez Ortega, F. Aminothiazole Ligand-Type Dioxo-Mo(VI) Complex Anchored on TiO2 Nanotubes for Selective Oxidation of Monoterpenes with Light and O2. Top. Catal. 2022, 65, 1088–1101. [Google Scholar] [CrossRef]
- Naróg, D.; Szczepanik, A.; Sobkowiak, A. Iron(II, III)-Catalyzed Oxidation of Limonene by Dioxygen. Catal. Lett. 2008, 120, 320–325. [Google Scholar] [CrossRef]
- Szczepanik, A.; Sobkowiak, A. Manganese(II)-Induced Oxidation of Limonene by Dioxygen. Catal. Lett. 2008, 126, 261–267. [Google Scholar] [CrossRef]
- Liu, J.; Ji, X.; Wang, C.; Wang, L.; Jian, P. Beneficial Synergistic Intermetallic Effect in ZnCo2O4 for Enhancing the Limonene Oxidation Catalysis. Inorg. Chem. 2023, 62, 18750–18757. [Google Scholar] [CrossRef]
- Ciriminna, R.; Parrino, F.; De Pasquale, C.; Palmisano, L.; Pagliaro, M. Photocatalytic partial oxidation of limonene to 1,2 limonene oxide. Chem. Commun. 2018, 54, 1008–1011. [Google Scholar] [CrossRef]
- Wang, W.; Agustin, D.; Poli, R. Influence of ligand substitution on molybdenum catalysts with tridentate Schiff base ligands for the organic solvent-free oxidation of limonene using aqueous TBHP as oxidant. Mol. Catal. 2017, 443, 52–59. [Google Scholar] [CrossRef]
- Yuan, L.S.; Chandren, S.; Efendi, J.O.N.; Ho, C.S.; Nur, H. Hydrophobic effect of silica functionalized with silylated Ti-salicylaldimine complex on limonene oxidation by aqueous hydrogen peroxide. J. Chem. Sci. 2015, 127, 1905–1917. [Google Scholar] [CrossRef]
- Kala Raj, N.K.; Puranik, V.G.; Gopinathan, C.; Ramaswamy, A.V. Selective oxidation of limonene over sodium salt of cobalt containing sandwich-type polyoxotungstate [WCo3(H2O)2{W9CoO34}2]10−. Appl. Catal. A Gen. 2003, 256, 265–273. [Google Scholar] [CrossRef]
- Godhani, D.R.; Nakum, H.D.; Parmar, D.K.; Mehta, J.P.; Desai, N.C. Zeolite-Y immobilized Metallo-ligand complexes: A novel heterogenous catalysts for selective oxidation. Inorg. Chem. Commun. 2016, 72, 105–116. [Google Scholar] [CrossRef]
- Oliveira, P.; Machado, A.; Ramos, A.M.; Fonseca, I.M.; Braz Fernandes, F.M.; Botelho do Rego, A.M.; Vital, J. Anchoring manganese acetylacetonate complex on MCM-41: Catalytic testing on limonene oxidation. Catal. Commun. 2007, 8, 1366–1372. [Google Scholar] [CrossRef]
- Bonon, A.J.; Kozlov, Y.N.; Bahú, J.O.; Filho, R.M.; Mandelli, D.; Shul’pin, G.B. Limonene epoxidation with H2O2 promoted by Al2O3: Kinetic study, experimental design. J. Catal. 2014, 319, 71–86. [Google Scholar] [CrossRef]
- Michel, T.; Cokoja, M.; Sieber, V.; Kühn, F.E. Selective epoxidation of (+)-limonene employing methyltrioxorhenium as catalyst. J. Mol. Catal. A Chem. 2012, 358, 159–165. [Google Scholar] [CrossRef]
- Młodzik, J.; Wróblewska, A.; Makuch, E.; Wróbel, R.J.; Michalkiewicz, B. Fe/EuroPh catalysts for limonene oxidation to 1,2-epoxylimonene, its diol, carveol, carvone and perillyl alcohol. Catal. Today 2016, 268, 111–120. [Google Scholar] [CrossRef]
- Wróblewska, A.; Makuch, E.; Młodzik, J.; Koren, Z.C.; Michalkiewicz, B. Oxidation of limonene over molybdenum dioxide-containing nanoporous carbon catalysts as a simple effective method for the utilization of waste orange peels. React. Kinet. Mech. Catal. 2018, 125, 843–858. [Google Scholar] [CrossRef]
- Modi, C.K.; Gade, B.G.; Chudasama, J.A.; Parmar, D.K.; Nakum, H.D.; Patel, A.L. Synthesis, spectral investigation and catalytic aspects of entrapped VO(IV) and Cu(II) complexes into the supercages of zeolite-Y. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 140, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.K.; Butani, P.M.; Thumar, N.J.; Jasani, P.M.; Padaliya, R.V.; Sandhiya, P.R.; Nakum, H.D.; Khan, M.N.; Makwana, D. Oxy-functionalization of olefins with neat and heterogenized binuclear V(IV)O and Fe(II) complexes: Effect of steric hindrance on product selectivity and output in homogeneous and heterogeneous phase. Mol. Catal. 2019, 474, 110424. [Google Scholar] [CrossRef]
- Santos, J.S.D.; Faria, A.L.; Amorin, P.M.; Luna, F.M.L.; Caiado, K.L.; Silva, D.O.; Sartoratto, P.P.; Assis, M.D. Iron(III) Porphyrin Covalently Supported onto Magnetic Amino-Functionalized Nanospheres as Catalyst for Hydrocarbon and Herbicide Oxidations. J. Braz. Chem. Soc. 2012, 23, 1411–1420. [Google Scholar] [CrossRef]
- Madadi, M.; Rahimi, R. Zeolite-immobilized Mn(III), Fe(III) and Co(III) complexes with 5,10,15,20-tetra(4-methoxyphenyl)porphyrin as heterogeneous catalysts for the epoxidation of (R)-(+)-limonene: Synthesis, characterization and catalytic activity. React. Kinet. Mech. Catal. 2012, 107, 215–229. [Google Scholar] [CrossRef]
- Modi, C.K.; Chudasama, J.A.; Nakum, H.D.; Parmar, D.K.; Patel, A.L. Catalytic oxidation of limonene over zeolite-Y entrapped oxovanadium (IV) complexes as heterogeneous catalysts. J. Mol. Catal. A Chem. 2014, 395, 151–161. [Google Scholar] [CrossRef]
- Godhani, D.R.; Nakum, H.D.; Parmar, D.K.; Mehta, J.P.; Desai, N.C. Zeolite Y encaged Ru(III) and Fe(III) complexes for oxidation of styrene, cyclohexene, limonene, and α-pinene: An eye-catching impact of H2SO4 on product selectivity. J. Mol. Catal. A Chem. 2017, 426, 223–237. [Google Scholar] [CrossRef]
- Mehta, J.P.; Parmar, D.K.; Nakum, H.D.; Godhani, D.R.; Desai, N.C. Enhanced catalytic oxidation of monoterpenes by zeolite-Y entrapped iron complex: Spectral studies and mechanistic vision. J. Porous Mater. 2018, 25, 1649–1658. [Google Scholar] [CrossRef]
- Nunes, M.S.; Gomes, D.M.; Gomes, A.C.; Neves, P.; Mendes, R.F.; Paz, F.A.A.; Lopes, A.D.; Pillinger, M.; Valente, A.A.; Gonçalves, I.S. A Molybdenum(VI) Complex of 5-(2-pyridyl-1-oxide)tetrazole: Synthesis, Structure, and Transformation into a MoO3-Based Hybrid Catalyst for the Epoxidation of Bio-Olefins. Catalysts 2023, 13, 565. [Google Scholar]
- Gawarecka, A.; Wróblewska, A. Limonene oxidation over Ti-MCM-41 and Ti-MWW catalysts with t-butyl hydroperoxide as the oxidant. React. Kinet. Mech. Catal. 2018, 124, 523–543. [Google Scholar] [CrossRef]
- Becerra, J.-A.; González, L.-M.; Villa, A.-L. A bio-inspired heterogeneous catalyst for the transformation of limonene from orange peel waste biomass into value-added products. Catal.Today 2018, 302, 250–260. [Google Scholar] [CrossRef]
- Abrantes, M.; Bruno, S.M.; Tomé, C.; Pillinger, M.; Gonçalves, I.S.; Valente, A.A. Epoxidation of DL-limonene using an indenyl molybdenum(II) tricarbonyl complex as catalyst precursor. Catal. Commun. 2011, 15, 64–67. [Google Scholar] [CrossRef]
- Oliveira, P.; Machado, A.; Ramos, A.M.; Fonseca, I.; Fernandes, F.M.B.; Rego, A.M.B.d.; Vital, J. MCM-41 anchored manganese salen complexes as catalysts for limonene oxidation. Microporous Mesoporous Mater. 2009, 120, 432–440. [Google Scholar] [CrossRef]
- Oliveira, P.; Ramos, A.M.; Fonseca, I.; Botelho do Rego, A.; Vital, J. Oxidation of limonene over carbon anchored transition metal Schiff base complexes: Effect of the linking agent. Catal. Today 2005, 102–103, 67–77. [Google Scholar]
- De Fátima Teixeira Gomes, M.; Antunes, O.A.C. Oxidation of limonene catalyzed by MnIII(Salen)Cl-H2O. Catal. Lett. 1996, 38, 133–134. [Google Scholar] [CrossRef]
- Cubillos, J.; Vásquez, S.; Montes de Correa, C. Salen manganese (III) complexes as catalysts for R-(+)-limonene oxidation. Appl. Catal. A Gen. 2010, 373, 57–65. [Google Scholar] [CrossRef]
- Lima, L.F.; Corraza, M.L.; Cardozo-Filho, L.; Márquez-Alvarez, H.; Antunes, O.A.C. Oxidation of limonene catalyzed by Metal(Salen) complexes. Braz. J. Chem. Eng. 2006, 23, 83–92. [Google Scholar] [CrossRef]
- Joseph, T.; Halligudi, S.B. Oxyfunctionalization of limonene using vanadium complex anchored on functionalized SBA-15. J. Mol. Catal. A Chem. 2005, 229, 241–247. [Google Scholar] [CrossRef]
- Gonçalves, J.A.; Bueno, A.C.; Gusevskaya, E.V. Palladium-catalyzed oxidation of monoterpenes: Highly selective syntheses of allylic ethers from limonene. J. Mol. Catal. A Chem. 2006, 252, 5–11. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; LDJayaweera, S.; ADias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Langa, E.; Valenzuela, A.; Ballestero, D.; Pino-Otín, M.R. Synergistic Activity of Thymol with Commercial Antibiotics against Critical and High WHO Priority Pathogenic Bacteria. Plants 2023, 12, 1868. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.L.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, N.; Abranches, D.O.; Silva, L.P.; Martins, M.A.R.; Carvalho, P.J.; Russina, O.; Triolo, A.; Paccou, L.; Guinet, Y.; Hedoux, A.; et al. Non-Ideality in Thymol + Menthol Type V Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2021, 9, 2203–2211. [Google Scholar] [CrossRef]
- Bergua, F.; Castro, M.; Lafuente, C.; Artal, M. Thymol+l-menthol eutectic mixtures: Thermophysical properties and possible applications as decontaminants. J. Mol. Liq. 2022, 368, 120789. [Google Scholar] [CrossRef]
- Benito, C.; Alcalde, R.; Atilhan, M.; Aparicio, S. High-pressure properties of type V Natural Deep Eutectic Solvents: The case of menthol: Thymol. J. Mol. Liq. 2023, 376, 121398. [Google Scholar] [CrossRef]
- Kamatou GP, P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, N.; Di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A.; Ghelardini, C. Menthol: A natural analgesic compound. Neurosci. Lett. 2002, 322, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Ohno, R.; Suzuki, N.; Tsugami, Y.; Nii, T.; Kobayashi, K.; Isobe, N. Menthol application on healthy and inflamed goat udders changes antimicrobial components in milk. Anim. Sci. J. 2023, 94, e13832. [Google Scholar] [CrossRef] [PubMed]
- Peel, J.; John, K.; Page, J.; Jeffries, O.; Heffernan, S.M.; Tallent, J.; Waldron, M. Topical application of isolated menthol and combined menthol-capsaicin creams: Exercise tolerance, thermal perception, pain, attentional focus and thermoregulation in the heat. Eur. J. Sport Sci. 2023, 23, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.M.; Ringer, K.L.; McConkey, M.E.; Croteau, R. Monoterpene metabolism. Cloning, expression, and characterization of menthone reductases from peppermint. Plant Physiol. 2005, 137, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Rydel-Ciszek, K.; Pacześniak, T.; Chmielarz, P.; Sobkowiak, A. Bio-Inspired Iron Pentadentate Complexes as Dioxygen Activators in the Oxidation of Cyclohexene and Limonene. Molecules 2023, 28, 2240. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I. Molecular Orbital Theory. In Molecular Orbitals and Organic Chemical Reactions; Wiley, John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 1–57. [Google Scholar]
- Ahmed, L.; Omer, R.; Qader, I.; Koparir, P. Theoretical Analysis of the Reactivity of Carmustine and Lomustine Drugs. J. Phys. Chem. Funct. Mater. 2022, 5, 84–96. [Google Scholar]
- Eryilmaz, S.; Gul, M.; Inkaya, E. Investigation of global reactivity descriptors of some perillaldehyde derivatives in different solvents by DFT method. Indian J. Chem. Technol. 2019, 26, 235–238. [Google Scholar]
- Mali, S.N.; Anand, A.; Zaki, M.E.A.; Al-Hussain, S.A.; Jawarkar, R.D.; Pandey, A.; Kuznetsov, A. Theoretical and Anti-Klebsiella pneumoniae Evaluations of Substituted 2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide and Imidazopyridine Hydrazide Derivatives. Molecules 2023, 28, 2801. [Google Scholar] [CrossRef]
- Boukabcha, N.; Benmohammed, A.; Belhachemi, M.H.M.; Goudjil, M.; Yahiaoui, S.; Megrouss, Y.; Djafri, A.; Khelloul, N.; Benyehlou, Z.D.; Djafri, A.; et al. Spectral investigation, TD-DFT study, Hirshfeld surface analysis, NCI-RDG, HOMO-LUMO, chemical reactivity and NLO properties of 1-(4-fluorobenzyl)-5-bromolindolin-2,3-dione. J. Mol. Struct. 2023, 1285, 135492. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. USA 1986, 83, 8440–8441. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Absolute electronegativity and hardness: Applications to organic chemistry. J. Org. Chem. 1989, 54, 1423–1430. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Subramanian, V.; Roy, D.R.; Chattaraj, P.K. Electrophilicity index as a possible descriptor of biological activity. Bioorganic Med. Chem. 2004, 12, 5533–5543. [Google Scholar] [CrossRef] [PubMed]
- Zaklika, J.; Hładyszowski, J.; Ordon, P. From the Electron Density Gradient to the Quantitative Reactivity Indicators: Local Softness and the Fukui Function. ACS Omega 2022, 7, 7745–7758. [Google Scholar] [CrossRef]
- Omer, R.; Koparir, P.; Qader, I.N.; Ahmed, L. Structure reactivity analysis for Phenylalanine and Tyrosine. Cumhur. Sci. J. 2021, 42, 576–585. [Google Scholar] [CrossRef]
- Giang, P.D.; Churchman, L.R.; Buczynski, J.B.; Bell, S.G.; Stok, J.E.; De Voss, J.J. CYP108N14: A Monoterpene Monooxygenase from Rhodococcus globerulus. Arch. Biochem. Biophys. 2024, 752, 109852. [Google Scholar] [CrossRef] [PubMed]
- Neaţu, F.; Culică, G.; Florea, M.; Parvulescu, V.I.; Cavani, F. Synthesis of Terephthalic Acid by p-Cymene Oxidation using Oxygen: Toward a More Sustainable Production of Bio-Polyethylene Terephthalate. ChemSusChem 2016, 9, 3102–3112. [Google Scholar] [CrossRef]
- Jakaria, M.; Cho, D.Y.; Ezazul Haque, M.; Karthivashan, G. Neuropharmacological Potential and Delivery Prospects of Thymoquinone for Neurological Disorders. Oxid. Med. Cell Longev. 2018, 2018, 1209801. [Google Scholar] [CrossRef]
- Liu, C.; Gao, Q.; Shang, Z.; Liu, J.; Zhou, S.; Dang, J.; Liu, L.; Lange, I.; Srividya, N.; Lange, B.M.; et al. Functional Characterization and Structural Insights Into Stereoselectivity of Pulegone Reductase in Menthol Biosynthesis. Front. Plant Sci. 2021, 12, 780970. [Google Scholar] [CrossRef] [PubMed]
- Kani, İ.; Taşkınlar, İ.; Uzel, Z.; Avan, İ. Catalytic oxidation of thymol and carvacrol with Mn(II)-benzoylbenzoate-bipyridine complex. Polyhedron 2024, 249, 116772. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, version 16, revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.J.; Scuseria, G.E. Predicting Band Gaps with Hybrid Density Functionals. J. Phys. Chem. Lett. 2016, 7, 4165–4170. [Google Scholar] [CrossRef] [PubMed]
- Moto Ongagna, J.; Tamafo Fouegue, A.D.; Ateba Amana, B.; Mouzong D’ambassa, G.; Zobo Mfomo, J.; Mbaze Meva’A, L.; Bikele Mama, D. B3LYP, M06 and B3PW91 DFT assignment of nd8 metal-bis-(N-heterocyclic carbene) complexes. J. Mol. Model. 2020, 26, 246. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Trung, N.Q.; Mechler, A.; Hoa, N.T.; Vo, Q.V. Calculating bond dissociation energies of X−H (X = C, N, O, S) bonds of aromatic systems via density functional theory: A detailed comparison of methods. R. Soc. Open Sci. 2022, 9, 220177. [Google Scholar] [CrossRef]




| Entry | Catalysts | Oxidants | Solvents | Oxidations Products | Ref. |
|---|---|---|---|---|---|
| 1 | MoCl2O2(Bipy)/TiO2-NT | O2/(λ = 360 nm) | MeCN | LO, DLO, CVN | [30] |
| 2 | MoO2(L1–L4)/TiO2-NT | O2/(λ = 360 nm) | MeCN | LO, DLO, CVN | [31,32] |
| 3 | CYP108N12 | O2 | Tris-HCl | PALC, PALD | [9] |
| 4 | [Fe(bpy)2]2+, [Fe(bpy)2]3+ | air, O2 | MeCN | LO, CVN, CVL, PALD | [33] |
| 5 | [Mn(bpy)2]2+ | air, O2 | MeCN | LO, CVN, CVL, PALD, PALC | [34] |
| 6 | TiO2-P25 | O2 | MeCN | LO | [36] |
| 7 | ZnCo2O4, isobutyraldehyde | O2 | MeCN | LO | [35] |
| 8 | Pd(OAc)2/PTSA/BQ, Na2PdCl4/PTSA/BQ | O2 | MeOH, ethanol, 2-Propanol | allylic ethers | [63] |
| 9 | NiAl-HT | O2 | Limonene | LO, CVN, CVL | [29] |
| 10 | [MoO2(SAP)]2, [MoO2(SATP)]2 | t-Bu-OOH | Limonene | LO, LDIOL | [37] |
| 11 | Ti:OTMS | H2O2 | Limonene | CVN, CVL, LO | [38] |
| 12 | Na10[Co5W19O70H4]·44H2O | air, H2O2 | MeCN, MeOH, acetone | LO, CVN, CVL | [39] |
| 13 | [M4(H2O)2(PW9O34)2]n−, M–CoII, MnII, FeIII | H2O2 | MeCN | LDIOL, CVN, CVL | [2] |
| 14 | [(C18H37)2N(CH3)2]3PW4O20, | H2O2 | Tire pyrolysis oil | LO, DLO, LDIOL | [10] |
| 15 | (nBu4N)3[NaHAsW9O33[P(O)R]2] (R = t-Bu or CH2CH2COOH) (n-Bu4N)3-[NaHPW9O34[As(O)p-C6H4NH2]2] | H2O2 | MeCN | LO, DLO, LDIOL | [11] |
| 16 | Co(II)-Y, Cu(II)-Y with Schiff base ligands | H2O2 | MeCN | CVN, CVL, LO, LDIOL | [40] |
| 17 | [Mn(acac)2APTS]@MCM-41 | H2O2 | Acetone–t-butanol | LO, CVL, CVN, polymer | [41] |
| 18 | Al2O3 | H2O2 | Ethyl acetate | LO, DLO, 8,9-LO | [42] |
| 19 | Ga(NO3)3, Al(NO3)3 | H2O2 | Ethyl acetate, THF | LO, DLO, LDIOL, 8,9-LO | [1] |
| 20 | MTO:L5-7 | H2O2 | CH2Cl2 | LO, 8,9-LO, DLO, CVL, CVN | [43] |
| 21 | carbon EuroPh with Fe | H2O2 | MeOH | PALC, CVL, CVN, LO, LDIOL | [44] |
| 22 | [VO(L8)H2O]-Y, [Cu(L8)H2O]-Y | H2O2 | MeCN | LDIOL, CVL, CVN, LO | [46] |
| 23 | [VO(sal2bz)]2, [VO(sal2bz)]2-Y [Fe(sal2bz)(H2O)2]2⋅2H2O, [Fe(sal2bz)(H2O)2]2-Y | H2O2 | MeCN | LDIOL, PALC, CVN, CVL | [47] |
| 24 | Mn(III)/Fe(III)/Co(III)/L9/Y/ammonium acetate | H2O2 | MeCN | LO, 8,9-LO | [49] |
| 25 | [FeII(L10)2(H2O)2]-Y | H2O2 | MeCN | CVN, CVL | [52] |
| 26 | RuY, FeY, 3Y–6Y | H2O2 | MeCN | CVN, CVL, LO, LDIOL | [51] |
| 27 | γ-Fe2O3/SiO2-NHFeP | m-CPBA, H2O2 | MeCN | LO, CVN, CVL | [48] |
| 28 | MoO2-EuroPh | H2O2, t-Bu-OOH | MeOH | CVN, CVL, LO, PALC | [45] |
| 29 | [VO(VFCH)2]-Y, [VO(VTCH)2]-Y, [VO(SFCH)·H2O]-Y, [VO(STCH)·H2O]-Y | H2O2, t-Bu-OOH | MeCN | LO, LDIOL, CVN, CVL | [50] |
| 30 | [MoO3(Hpto)]∙H2O | t-Bu-OOH | α,α,α- trifluorotoluene | LO, LDIOL, DLO | [53] |
| 31 | [MoO3(Hpytz)] | t-Bu-OOH | LO, LDIOL, DLO | [26] | |
| 32 | Ti-MCM-41, Ti-MWW | t-Bu-OOH | MeOH | LO, CVN, CVL, PALC | [54] |
| 33 | FePcCl16-NH2-SiO2 | t-Bu-OOH | Acetone | CVN, LO, CVL | [55] |
| 34 | cobalt(II)-(acac)-carbon-based catalysts | t-Bu-OOH | Acetone–t-butanol | LO, CVN, CVL, polymer | [58] |
| 35 | (η5-C9H7)Mo(CO)3Me | t-Bu-OOH | Decane, t-butanol | LO, DLO, LDIOL | [56] |
| 36 | MCM-41-Mn(4-OHsalen), MCM-41Mn(4-OHsalhd), MCM-41 Mn(4-OHsalophen) | t-Bu-OOH | Acetone–t-butanol | LO, CVN, CVL, polymer | [57] |
| 37 | Mn(III)-Jacobsen-type catalysts | KHSO5 | Acetone–H2O | DLO | [60] |
| 38 | Mn(Salen)Cl∙H2O | PhIO | CH2Cl2 | LO, CVN, PALD | [59] |
| 39 | M(Salen)Cl∙H2O M = MnII, NiII, CoII | PhIO, NaOCl | Acetone, MeCN, CH2Cl2, ethyl acetate | LO, CVN, CVL | [61] |
| 40 | VO(Salten)-SBA-15 | UHP | MeCN | LO, CVN, CVL, carvacrol | [62] |
| Gas | H2O | MeCN | MeOH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EHOMO, [eV] | ELUMO, [eV] | Egap, [eV] | EHOMO, [eV] | ELUMO, [eV] | Egap, [eV] | EHOMO, [eV] | ELUMO, [eV] | Egap, [eV] | EHOMO, [eV] | ELUMO, [eV] | Egap, [eV] | |
| limonene | −6.41867 | −0.02367 | 6.39500 | −6.14057 | 0.29361 | 6.43418 | −6.14411 | 0.28890 | 6.43309 | −6.14492 | 0.28790 | 6.43282 |
| cymene | −6.45105 | −0.32817 | 6.12288 | −6.22846 | −0.11647 | 6.11199 | −6.23010 | −0.11783 | 6.11227 | −6.23037 | −0.11810 | 6.11227 |
| pinene | −6.20724 | 0.02748 | 6.23472 | −5.91145 | 0.33198 | 6.24343 | −5.91553 | 0.32708 | 6.24261 | −5.91635 | 0.32627 | 6.24262 |
| thymol | −6.08669 | −0.39212 | 5.69457 | −5.83036 | −0.11211 | 5.71825 | −5.83362 | −0.11701 | 5.71661 | −5.84614 | −0.12789 | 5.71825 |
| menthol | −7.27230 | −0.03837 | 7.23393 | −7.03801 | 0.37089 | 7.40890 | −7.04209 | 0.363554 | 7.40564 | −7.04263 | 0.36219 | 7.40482 |
| Terpene | E0 [a.u.] | I [eV] | A [eV] | X [eV] | η [eV] | S [eV] | ω [eV] | μ [eV] | |
|---|---|---|---|---|---|---|---|---|---|
| limonene | −390.76016952 | 6.419 | 0.024 | 3.221 | 3.197 | 0.156 | 1.623 | −3.221 | |
| cymene | −389.59360262 | 6.451 | 0.328 | 3.390 | 3.061 | 0.163 | 1.876 | −3.390 | |
| pinene | −390.74018343 | 6.207 | −0.027 | 3.090 | 3.117 | 0.160 | 1.531 | −3.090 | |
| thymol | −464.83540277 | 6.087 | 0.392 | 3.239 | 2.847 | 0.176 | 1.843 | −3.239 | |
| menthol | −468.44884783 | 7.272 | 0.038 | 3.655 | 3.617 | 0.138 | 1.847 | −3.655 | |
| MeCN | limonene | −390.76198136 | 6.144 | −0.289 | 2.928 | 3.217 | 0.155 | 1.332 | −2.928 |
| cymene | −389.59597035 | 6.230 | 0.118 | 3.174 | 3.056 | 0.164 | 1.648 | −3.174 | |
| pinene | −390.74104612 | 5.916 | −0.327 | 2.794 | 3.121 | 0.160 | 1.251 | −2.794 | |
| thymol | −464.84156521 | 5.834 | 0.117 | 2.975 | 2.858 | 0.175 | 1.549 | −2.975 | |
| menthol | −468.45328899 | 7.042 | −0.364 | 3.339 | 3.703 | 0.135 | 1.506 | −3.339 | |
| H2O | limonene | −390.76203510 | 6.141 | −0.293 | 2.923 | 3.217 | 0.155 | 1.328 | −2.923 |
| cymene | −389.59604600 | 6.228 | 0.116 | 3.172 | 3.056 | 0.164 | 1.647 | −3.172 | |
| pinene | −390.74107098 | 5.911 | −0.332 | 2.790 | 3.122 | 0.160 | 1.247 | −2.790 | |
| thymol | −464.84176291 | 5.830 | 0.112 | 2.971 | 2.859 | 0.175 | 1.544 | −2.971 | |
| menthol | −468.45341693 | 7.038 | −0.371 | 3.334 | 3.705 | 0.135 | 1.500 | −3.334 | |
| MeOH | limonene | −390.76197221 | 6.145 | −0.288 | 2.929 | 3.216 | 0.155 | 1.333 | −2.929 |
| cymene | −389.59595751 | 6.230 | 0.118 | 3.174 | 3.056 | 0.164 | 1.648 | −3.174 | |
| pinene | −390.74104188 | 5.916 | −0.326 | 2.795 | 3.121 | 0.160 | 1.251 | −2.795 | |
| thymol | −464.84153739 | 5.846 | 0.128 | 2.987 | 2.859 | 0.175 | 1.560 | −2.987 | |
| menthol | −468.45326721 | 7.043 | −0.362 | 3.340 | 3.702 | 0.135 | 1.507 | −3.340 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydel-Ciszek, K. DFT Studies of the Activity and Reactivity of Limonene in Comparison with Selected Monoterpenes. Molecules 2024, 29, 1579. https://doi.org/10.3390/molecules29071579
Rydel-Ciszek K. DFT Studies of the Activity and Reactivity of Limonene in Comparison with Selected Monoterpenes. Molecules. 2024; 29(7):1579. https://doi.org/10.3390/molecules29071579
Chicago/Turabian StyleRydel-Ciszek, Katarzyna. 2024. "DFT Studies of the Activity and Reactivity of Limonene in Comparison with Selected Monoterpenes" Molecules 29, no. 7: 1579. https://doi.org/10.3390/molecules29071579