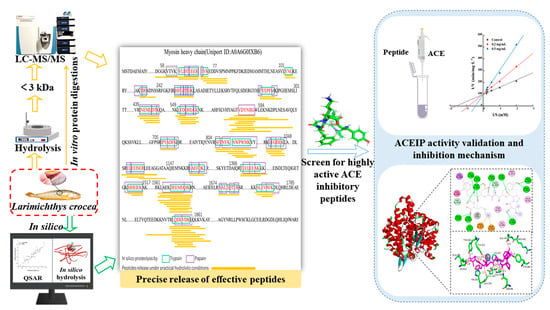
Biodirected Screening and Preparation of Larimichthys crocea Angiotensin-I-Converting Enzyme-Inhibitory Peptides by a Combined In Vitro and In Silico Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of ACE-Inhibitory Activity of Different Molecular Weight Fractions in Larimichthys crocea Protein Enzymatic Hydrolysates
2.2. QSAR Prediction Model
2.3. Identification of Potential ACE-Inhibitory Peptides
2.4. Prediction and Screening of ACE-Inhibitory Peptide Activity
2.5. Validation of ACE-Inhibitory Peptide Activity In Vitro
2.6. Stability Analysis of ACE-Inhibitory Peptides
2.7. Inhibition Kinetics Study
2.8. Molecular Interactions between Peptides and ACE
3. Materials and Methods
3.1. Materials
3.2. In Silico Hydrolysis
3.3. Preparation of Larimichthys crocea Protein Extract
3.4. Determination of ACE-Inhibitory Activity
3.5. QSAR Modeling to Evaluate Potential ACE-Inhibitory Peptides in Larimichthys crocea Protein
3.6. Nano LC-MS/MS Analysis
3.7. Synthesis of Peptides
3.8. The Stabilization of ACE-Inhibitory Peptides during Simulated Digestion In Vitro
3.9. Inhibitory Kinetics Study
3.10. Molecular Docking
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gai, Z.; Hu, S.; Gong, G.; Zhao, J. Recent Advances in Understanding Dietary Polyphenols Protecting against Hypertension. Trends Food Sci. Technol. 2023, 138, 685–696. [Google Scholar] [CrossRef]
- Dang, Y.; Zhou, T.; Hao, L.; Cao, J.; Sun, Y.; Pan, D. In Vitro and in Vivo Studies on the Angiotensin-Converting Enzyme Inhibitory Activity Peptides Isolated from Broccoli Protein Hydrolysate. J. Agric. Food Chem. 2019, 67, 6757–6764. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, C.; Sun-Waterhouse, D.; Zhao, T.; Waterhouse, G.I.N.; Zhao, M.; Su, G. Identification of Post-Digestion Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptides from Soybean Protein Isolate: Their Production Conditions and in Silico Molecular Docking with ACE. Food Chem. 2021, 345, 128855. [Google Scholar] [CrossRef]
- Yang, D.; Li, L.; Li, C.; Chen, S.; Deng, J.; Yang, S. Formation and Inhibition Mechanism of Novel Angiotensin I Converting Enzyme Inhibitory Peptides from Chouguiyu. Front. Nutr. 2022, 9, 920945. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Comer, J.; Li, Y. Bioinformatics Approaches to Discovering Food-Derived Bioactive Peptides: Reviews and Perspectives. Trac-Trends Anal. Chem. 2023, 162, 117051. [Google Scholar] [CrossRef]
- Ibarz-Blanch, N.; Alcaide-Hidalgo, J.M.; Cortés-Espinar, A.J.; Albi-Puig, J.; Suárez, M.; Mulero, M.; Morales, D.; Bravo, F.I. Chicken Slaughterhouse By-Products: A Source of Protein Hydrolysates to Manage Non-Communicable Diseases. Trends Food Sci. Technol. 2023, 139, 104125. [Google Scholar] [CrossRef]
- Tu, M.; Cheng, S.; Lu, W.; Du, M. Advancement and Prospects of Bioinformatics Analysis for Studying Bioactive Peptides from Food-Derived Protein: Sequence, Structure, and Functions. Trac-Trends Anal. Chem. 2018, 105, 7–17. [Google Scholar] [CrossRef]
- Ngoh, Y.-Y.; Gan, C.-Y. Identification of Pinto Bean Peptides with Inhibitory Effects on α-Amylase and Angiotensin Converting Enzyme (ACE) Activities Using an Integrated Bioinformatics-Assisted Approach. Food Chem. 2018, 267, 124–131. [Google Scholar] [CrossRef]
- Li, R.; Zhou, X.; Sun, L.; Zhuang, Y. Identification, in Silico Screening, and Molecular Docking of Novel ACE Inhibitory Peptides Isolated from the Edible Symbiot Boletus Griseus-Hypomyces Chrysospermus. LWT-Food Sci. Technol. 2022, 169, 114008. [Google Scholar] [CrossRef]
- Udenigwe, C.C. Bioinformatics Approaches, Prospects and Challenges of Food Bioactive Peptide Research. Trends Food Sci. Technol. 2014, 36, 137–143. [Google Scholar] [CrossRef]
- Lee, S.-H.; Qian, Z.-J.; Kim, S.-K. A Novel Angiotensin I Converting Enzyme Inhibitory Peptide from Tuna Frame Protein Hydrolysate and Its Antihypertensive Effect in Spontaneously Hypertensive Rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Fu, B.; Wu, D.; Cheng, S.; Xu, X.; Zhang, L.; Wang, L.; El-Seedi, H.R.; Liu, H.; Du, M. Three Novel Umami Peptides Derived from the Alcohol Extract of the Pacific Oyster (Crassostrea Gigas): Identification, Characterizations and Interactions with T1R1/T1R3 Taste Receptors. Food Sci. Hum. Wellness 2024, 13, 146–153. [Google Scholar] [CrossRef]
- Tsai, J.-S.; Chen, J.-L.; Pan, B.S. ACE-Inhibitory Peptides Identified from the Muscle Protein Hydrolysate of Hard Clam (Meretrix Lusoria). Process Biochem. 2008, 43, 743–747. [Google Scholar] [CrossRef]
- Hayes, M.; Mora, L.; Hussey, K.; Aluko, R.E. Boarfish Protein Recovery Using the pH-Shift Process and Generation of Protein Hydrolysates with ACE-I and Antihypertensive Bioactivities in Spontaneously Hypertensive Rats. Innov. Food Sci. Emerg. Technol. 2016, 37, 253–260. [Google Scholar] [CrossRef]
- Ao, J.; Mu, Y.; Xiang, L.-X.; Fan, D.; Feng, M.; Zhang, S.; Shi, Q.; Zhu, L.-Y.; Li, T.; Ding, Y.; et al. Genome Sequencing of the Perciform Fish Larimichthys Crocea Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation. PLoS Genet. 2015, 11, e1005118. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, Y.; Zhao, Y.; Zhu, H.; Zhang, Y.; Zhao, Y.; Liu, X. Comparative Transcriptome Analysis Reveals Immunoregulation Mechanism in the Spleen of Large Yellow Croaker (Larimichthys Crocea) in Response to Vibrio Harveyi Infection. Aquac. Rep. 2023, 31, 101657. [Google Scholar] [CrossRef]
- Liang, P.; Cheng, X.; Xu, Y.; Cheng, W.; Chen, L. Determination of Fatty Acid Composition and Phospholipid Molecular Species of Large Yellow Croaker (Pseudosciaena Crocea) Roe from China. J. Aquat. Food Prod. Technol. 2017, 26, 1259–1265. [Google Scholar] [CrossRef]
- Mu, H.; Wei, Z.; Yi, L.; Liang, H.; Zhao, L.; Zhang, W.; Mai, K. Dietary Fishmeal Levels Affect the Volatile Compounds in Cooked Muscle of Farmed Large Yellow Croaker Larimichthys Crocea. Aquac. Res. 2017, 48, 5821–5834. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, C.; Chen, Y.; Zheng, B. Purification and Characterization of Antioxidant Peptides of Pseudosciaena Crocea Protein Hydrolysates. Molecules 2017, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, S.; Wang, X.; Xie, R.; Cheng, J.; He, T.; Chen, X.; Zhang, X.-Y. A Synthetic Peptide Based on Large Yellow Croaker (Larimichthys Crocea) IFNG1R Protein Sequence Has Potential Antimicrobial Activity against Pseudomonas Plecoglossicida. Front. Mar. Sci. 2022, 9, 1038013. [Google Scholar] [CrossRef]
- Zheng, L.; Qiu, J.; Liu, H.; Chi, C.; Lin, L. Potential Anticancer Activity Analysis of Piscidin 5-like from Larimichthys Crocea. Acta Oceanol. Sin. 2022, 41, 53–60. [Google Scholar] [CrossRef]
- Bhadkaria, A.; Narvekar, D.T.; Nagar, D.P.; Sah, S.P.; Srivastava, N.; Bhagyawant, S.S. Purification, Molecular Docking and in Vivo Analyses of Novel Angiotensin-Converting Enzyme Inhibitory Peptides from Protein Hydrolysate of Moth Bean (Vigna Aconitifolia (Jacq.) Marechal) Seeds. Int. J. Biol. Macromol. 2023, 230, 123138. [Google Scholar] [CrossRef]
- Duan, X.; Dong, Y.; Zhang, M.; Li, Z.; Bu, G.; Chen, F. Identification and Molecular Interactions of Novel ACE Inhibitory Peptides from Rapeseed Protein. Food Chem. 2023, 422, 136085. [Google Scholar] [CrossRef]
- Hu, X.; Dai, Z.; Jin, R. Purification and Identification of a Novel Angiotensin Converting Enzyme Inhibitory Peptide from the Enzymatic Hydrolysate of Lepidotrigla Microptera. Foods 2022, 11, 1889. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Young, J.F.; Lokke, M.M.; Lametsch, R.; Aluko, R.E.; Therkildsen, M. Revalorisation of Bovine Collagen as a Potential Precursor of Angiotensin 1-Converting Enzyme (ACE) Inhibitory Peptides Based on in Silico and in Vitro Protein Digestions. J. Funct. Food. 2016, 24, 196–206. [Google Scholar] [CrossRef]
- Wu, J.P.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure-Activity Relationship Study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhang, L.; Han, X.; Cheng, D. Novel Angiotensin I-Converting Enzyme Inhibitory Peptides from Protease Hydrolysates of Qula Casein: Quantitative Structure-Activity Relationship Modeling and Molecular Docking Study. J. Funct. Food. 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Shao, M.; Wu, H.; Wang, B.; Zhang, X.; Gao, X.; Jiang, M.; Su, R.; Shen, X. Identification and Characterization of Novel ACE Inhibitory and Antioxidant Peptides from Sardina Pilchardus Hydrolysate. Foods 2023, 12, 2216. [Google Scholar] [CrossRef]
- Zaharuddin, N.D.; Barkia, I.; Ibadullah, W.Z.W.; Zarei, M.; Saari, N. Identification, Molecular Docking, and Kinetic Studies of Six Novel Angiotensin-I-Converting Enzyme (ACE) Inhibitory Peptides Derived from Kenaf (Hibiscus Cannabinus L.) Seed. Int. J. Biol. Macromol. 2022, 220, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Liu, D.; Xie, H.; Zhang, X.; Han, X.; Lv, Z. Screening and Mechanism of Novel Angiotensin-I-Converting Enzyme Inhibitory Peptides in X. Sorbifolia Seed Meal: A Computer-Assisted Experimental Study Method. Molecules 2022, 27, 8792. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Wang, H.; Hu, Z.; Xie, X.; Chen, H.; Tu, Z. Identification of Novel Angiotensin Converting Enzyme (ACE) Inhibitory Peptides from Pacific Saury: In Vivo Antihypertensive Effect and Transport Route. Int. J. Biol. Macromol. 2024, 254, 127196. [Google Scholar] [CrossRef]
- Zheng, S.-L.; Luo, Q.-B.; Suo, S.-K.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Preparation, Identification, Molecular Docking Study and Protective Function on HUVECs of Novel ACE Inhibitory Peptides from Protein Hydrolysate of Skipjack Tuna Muscle. Mar. Drugs 2022, 20, 176. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Malomo, S.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and Functional Characterization of Hemp Seed (Cannabis Sativa L.) Protein-Derived Antioxidant and Antihypertensive Peptides. J. Funct. Food. 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Martin-Martinez, M.; Angeles Bonache, M.; Gonzalez-Muniz, R.; Penas, E.; Frias, J.; Martinez-Villaluenga, C. Identification, Functional Gastrointestinal Stability and Molecular Docking Studies of Lentil Peptides with Dual Antioxidant and Angiotensin I Converting Enzyme Inhibitory Activities. Food Chem. 2017, 221, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Yang, W.; Zhu, Q.; Zhang, G.; Zhang, X.; Liu, L.; Li, X.; Hussain, M.; Ni, C.; Jiang, X. Proteolysis and ACE-Inhibitory Peptide Profile of Cheddar Cheese: Effect of Digestion Treatment and Different Probiotics. LWT-Food Sci. Technol. 2021, 145, 111295. [Google Scholar] [CrossRef]
- Ye, S.; Chen, Q.; Li, D.; Zhou, H.; Chen, Y.; Meng, C.; Hong, J. Isolation and Identification of Novel Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Pony Seed and Evaluation of the Inhibitory Mechanisms. J. Funct. Food. 2022, 95, 105151. [Google Scholar] [CrossRef]
- Wongngam, W.; Hamzeh, A.; Tian, F.; Roytrakul, S.; Yongsawatdigul, J. Purification and Molecular Docking of Angiotensin Converting Enzyme-Inhibitory Peptides Derived from Corn Gluten Meal Hydrolysate and from in Silico Gastrointestinal Digestion. Process Biochem. 2023, 129, 113–120. [Google Scholar] [CrossRef]
- Sasaki, C.; Tamura, S.; Tohse, R.; Fujita, S.; Kikuchi, M.; Asada, C.; Nakamura, Y. Isolation and Identification of an Angiotensin I-Converting Enzyme Inhibitory Peptide from Pearl Oyster (Pinctada Fucata) Shell Protein Hydrolysate. Process Biochem. 2019, 77, 137–142. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, J.; Guo, J.; Liu, J.; Xia, Z.; Chen, B.; Ma, F.; Cao, Y. Two Novel Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Rice (Oryza Sativa L.) Bran Protein. J. Agric. Food Chem. 2023, 71, 4153–4162. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, W.; Zhang, Y.-Q.; Dai, Z.-Y. A Novel Angiotensin-Converting Enzyme (ACE) Inhibitory Peptide from Tilapia Skin: Preparation, Identification and Its Potential Antihypertensive Mechanism. Food Chem. 2024, 430, 137074. [Google Scholar] [CrossRef]
- Wei, D.; Fan, W.; Xu, Y. Identification of Water-Soluble Peptides in Distilled Spent Grain and Its Angiotensin Converting Enzyme (ACE) Inhibitory Activity Based on UPLC-Q-TOF-MS and Proteomics Analysis. Food Chem. 2021, 353, 129521. [Google Scholar] [CrossRef]
- Shao, B.; Huang, X.; Xu, M.; Cheng, D.; Li, X.; Li, M. Peptides Isolated from Black Soybean Synergistically Inhibit the Activity of Angiotensin Converting Enzyme (ACE). J. Funct. Foods 2023, 106, 105604. [Google Scholar] [CrossRef]
- Ma, T.; Fu, Q.; Mei, Q.; Tu, Z.; Zhang, L. Extraction Optimization and Screening of Angiotensin-Converting Enzyme Inhibitory Peptides from Channa Striatus through Bioaffinity Ultrafiltration Coupled with LC-Orbitrap-MS/MS and Molecular Docking. Food Chem. 2021, 354, 129589. [Google Scholar] [CrossRef]
- Cao, M.J.; Jiang, X.J.; Zhong, H.C.; Zhang, Z.J.; Su, W.J. Degradation of Myofibrillar Proteins by a Myofibril-Bound Serine Proteinase in the Skeletal Muscle of Crucian Carp (Carasius Auratus). Food Chem. 2006, 94, 7–13. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Zhong, Q.; Wu, Y.; Xia, W. Purification and Characterization of a Novel Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptide Derived from Enzymatic Hydrolysate of Grass Carp Protein. Peptides 2012, 33, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-C.; Wang, J.; Zheng, B.-D.; Pang, J.; Chen, L.-J.; Lin, H.-T.; Guo, X. Simultaneous Determination of 8 Small Antihypertensive Peptides with Tyrosine at the C-Terminal in Laminaria Japonica Hydrolysates by Rp-Hplc Method. J. Food Process Preserv. 2016, 40, 492–501. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, L.; Han, X.; Meng, Z.; Zhang, J.; Wu, Y.; Cheng, D. Quantitative Structure-Activity Relationship Modeling Coupled with Molecular Docking Analysis in Screening of Angiotensin I-Converting Enzyme Inhibitory Peptides from Qula Casein Hydrolysates Obtained by Two-Enzyme Combination Hydrolysis. J. Agric. Food Chem. 2018, 66, 3221–3228. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xue, J.; Wang, P.; Wang, X.; Zhang, J.; Fang, X.; He, Z.; Wu, F. Identification of a Novel ACE Inhibitory Hexapeptide from Camellia Seed Cake and Evaluation of Its Stability. Foods 2023, 12, 501. [Google Scholar] [CrossRef]





| Peptides | IC50 (µM) | Peptides | IC50 (µM) | Peptides | IC50 (µM) |
|---|---|---|---|---|---|
| TVK | 205.63 | NWPWMK | 8.19 | AALEQTER | 37.25 |
| TIR | 219.84 | IPYADFK | 0.64 | INEMLDTK | 1.82 |
| YNL | 43.40 | SIHEIEK | 23.51 | DEEMEQIK | 30.98 |
| EAEFQK | 416.43 | LQDLVDK | 112.97 | NLEVAVK | 11.29 |
| FYEPFM | 9.53 | VDYNIIG | 15.26 | VLDTEEER | 16.77 |
| SFTNVK | 52.93 | TEELEEAK | 12.74 | IHFGTTGK | 7.87 |
| LEGDLK | 233.12 | LYDQHLGK | 3.25 | ELEEISER | 56.22 |
| Peptides | IPYADFK | LYDHLGK | INEMLDTK | IHFGTTGK | NWPWMK | FYEPFM |
|---|---|---|---|---|---|---|
| IC50 value before digestion (µM) | 0.63 ± 0.09 a | 9.41 ± 0.24 b | 6.53 ± 0.21 b | 5.34 ± 0.15 b | 6.23 ± 0.34 a | 10.26 ± 0.21 a |
| IC50 value after digestion (µM) | 0.80 ± 0.08 a | 24.34 ± 0.29 a | 15.65 ± 0.15 a | 33.73 ± 0.23 a | 7.27 ± 0.17 a | 11.41 ± 0.43 a |
| Abbrev. | Name | z1 | z2 | z3 | z4 | z5 |
|---|---|---|---|---|---|---|
| Ala | A | 0.24 | −2.32 | 0.60 | −0.14 | 1.30 |
| Arg | R | 3.52 | 2.50 | −3.50 | 1.99 | −0.17 |
| Asn | N | 3.05 | 1.62 | 1.04 | −1.15 | 1.61 |
| Asp | D | 3.98 | 0.93 | 1.93 | −2.46 | 0.75 |
| Cys | C | 0.84 | −1.67 | 3.71 | 0.18 | −2.65 |
| Gln | Q | 1.75 | 0.50 | −1.44 | −1.34 | 0.66 |
| Glu | E | 3.11 | 0.26 | −0.11 | −3.04 | −0.25 |
| Gly | G | 2.05 | −4.06 | 0.36 | −0.82 | −0.38 |
| His | H | 2.47 | 1.95 | 0.26 | 3.90 | 0.09 |
| Ile | I | −3.89 | −1.73 | −1.71 | −0.84 | 0.26 |
| Leu | L | −4.28 | −1.30 | −1.49 | −0.72 | 0.84 |
| Lys | K | 2.29 | 0.89 | −2.49 | 1.49 | 0.31 |
| Met | M | −2.85 | −0.22 | 0.47 | 1.94 | −0.98 |
| Phe | F | −4.22 | 1.94 | 1.06 | 0.54 | −0.62 |
| Pro | P | −1.66 | 0.27 | 1.84 | 0.70 | 2.00 |
| Ser | S | 2.39 | −1.07 | 1.15 | −1.39 | 0.67 |
| Thr | T | 0.75 | −2.18 | −1.12 | −1.46 | −0.40 |
| Trp | W | −4.36 | 3.94 | 0.59 | 3.44 | −1.59 |
| Tyr | Y | −2.54 | 2.44 | 0.43 | 0.04 | −1.47 |
| Val | V | −2.59 | −2.64 | −1.54 | −0.85 | −0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Wang, C.; Huang, B.; Chen, Y.; Liu, Z.; Chen, H.; Chen, J. Biodirected Screening and Preparation of Larimichthys crocea Angiotensin-I-Converting Enzyme-Inhibitory Peptides by a Combined In Vitro and In Silico Approach. Molecules 2024, 29, 1134. https://doi.org/10.3390/molecules29051134
Yang Z, Wang C, Huang B, Chen Y, Liu Z, Chen H, Chen J. Biodirected Screening and Preparation of Larimichthys crocea Angiotensin-I-Converting Enzyme-Inhibitory Peptides by a Combined In Vitro and In Silico Approach. Molecules. 2024; 29(5):1134. https://doi.org/10.3390/molecules29051134
Chicago/Turabian StyleYang, Zhizhi, Changrong Wang, Baote Huang, Yihui Chen, Zhiyu Liu, Hongbin Chen, and Jicheng Chen. 2024. "Biodirected Screening and Preparation of Larimichthys crocea Angiotensin-I-Converting Enzyme-Inhibitory Peptides by a Combined In Vitro and In Silico Approach" Molecules 29, no. 5: 1134. https://doi.org/10.3390/molecules29051134