A Molecularly Imprinted Electrochemical Sensor Based on TiO2@Ti3C2Tx for Highly Sensitive and Selective Detection of Chlortetracycline
Abstract
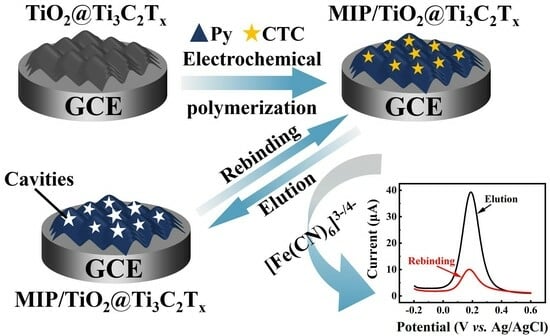
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Materials
2.2. Electrochemical Characterizations
2.3. Optimization of Experimental Parameters
2.4. The Performance of MIP/TiO2@Ti3C2Tx/GCE for Detecting CTC
2.5. Reproducibility, Repeatability, Stability, and Selectivity Research
2.6. Real Sample Analysis
3. Experimental Section
3.1. Reagent
3.2. Apparatus
3.3. Synthesis of TiO2@Ti3C2Tx
3.4. Preparation of TiO2@Ti3C2Tx/GCE and MIP/TiO2@Ti3C2Tx/GCE
3.5. Electrochemical Measurements
3.6. Preparation of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leichtweis, J.; Vieira, Y.; Welter, N.; Silvestri, S.; Dotto, G.L.; Carissimi, E. A review of the occurrence, disposal, determination, toxicity and remediation technologies of the tetracycline antibiotic. Process Saf. Environ. 2022, 160, 25–40. [Google Scholar] [CrossRef]
- Wang, X.S.; Zhang, S. A highly selective fluorescent sensor for chlortetracycline based on histidine-templated copper nanoclusters. Spectrochim. Acta Part A 2022, 281, 121588–121595. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jia, P.; Hou, J.; Zhao, S.; Wang, L. An ingenious turn-on ratiometric fluorescence sensor for sensitive and visual detection of tetracyclines. Food Chem. 2022, 396, 133693–133700. [Google Scholar] [CrossRef]
- Phomai, K.; Supharoek, S.A.; Vichapong, J.; Grudpan, K.; Ponhong, K. One-pot co-extraction of dispersive solid phase extraction employing iron-tannic nanoparticles assisted cloud point extraction for the determination of tetracyclines by high-performance liquid chromatography. Talanta 2023, 252, 123852–123861. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.; Zhang, Y.; Kang, Y.; Hu, H.; Iqbal, J.; Du, Y. Rapid determination of illegal additives chrysoidin and malachite green by surface-enhanced Raman scattering with silanized support based substrate. Chin. Chem. Lett. 2018, 29, 981–984. [Google Scholar] [CrossRef]
- Sheng, W.; Chang, Q.; Shi, Y.; Duan, W.; Zhang, Y.; Wang, S. Visual and fluorometric lateral flow immunoassay combined with a dual-functional test mode for rapid determination of tetracycline antibiotics. Microchim. Acta 2018, 185, 404–413. [Google Scholar] [CrossRef]
- Pang, Y.H.; Lv, Z.Y.; Sun, J.C.; Yang, C.; Shen, X.F. Collaborative compounding of metal-organic frameworks for dispersive solid-phase extraction HPLC-MS/MS determination of tetracyclines in honey. Food Chem. 2021, 355, 129411–129417. [Google Scholar] [CrossRef]
- Yu, L.; Chen, H.; Yue, J.; Chen, X.; Sun, M.; Tan, H.; Asiri, A.M.; Alamry, K.A.; Wang, X.; Wang, S. Metal-Organic Framework Enhances Aggregation-Induced Fluorescence of Chlortetracycline and the Application for Detection. Anal. Chem. 2019, 91, 5913–5921. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wen, M.J.; Zhang, Y.S.; Guo, Z.S.; Bai, X.; Song, J.X.; Liu, P.; Wang, Y.Y.; Li, J.L. Multiple fluorescence response behaviors towards antibiotics and bacteria based on a highly stable Cd-MOF. J. Hazard. Mater. 2022, 423 Pt B, 127132–127141. [Google Scholar] [CrossRef]
- Long, C.; Deng, B.; Sun, S.; Meng, S. Simultaneous determination of chlortetracycline, ampicillin and sarafloxacin in milk using capillary electrophoresis with electrochemiluminescence detection. Food Addit. Contam. A 2017, 34, 24–31. [Google Scholar] [CrossRef]
- Song, J.; Huang, M.; Lin, X.; Li, S.F.Y.; Jiang, N.; Liu, Y.; Guo, H.; Li, Y. Novel Fe-based metal–organic framework (MOF) modified carbon nanofiber as a highly selective and sensitive electrochemical sensor for tetracycline detection. Chem. Eng. J. 2022, 427, 130913–130926. [Google Scholar] [CrossRef]
- Pakapongpan, S.; Poo-Arporn, Y.; Tuantranont, A.; Poo-Arporn, R.P. A facile one-pot synthesis of magnetic iron oxide nanoparticles embed N-doped graphene modified magnetic screen printed electrode for electrochemical sensing of chloramphenicol and diethylstilbestrol. Talanta 2022, 241, 123184–123195. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Abnous, K. A novel M-shape electrochemical aptasensor for ultrasensitive detection of tetracyclines. Biosens. Bioelectron. 2016, 85, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Majdinasab, M.; Hayat, A. A perspective on non-enzymatic electrochemical nanosensors for direct detection of pesticides. Curr. Opin. Electrochem. 2018, 11, 12–18. [Google Scholar] [CrossRef]
- Govindaraj, M.; Srivastava, A.; Muthukumaran, M.K.; Tsai, P.C.; Lin, Y.C.; Raja, B.K.; Rajendran, J.; Ponnusamy, V.K.; Arockia Selvi, J. Current advancements and prospects of enzymatic and non-enzymatic electrochemical glucose sensors. Int. J. Biol. Macromol. 2023, 253 Pt 2, 126680–126708. [Google Scholar] [CrossRef]
- Shao, Y.; Zheng, R.; Wang, P.; Li, Y.; Zhao, Z.; An, J.; Hao, C.; Kang, M. A novel surface molecularly imprinted polymer electrochemical sensor based on porous magnetic TiO2 for highly sensitive and selective detection of tetracycline. Environ. Sci. Nano 2023, 10, 1614–1628. [Google Scholar] [CrossRef]
- Han, S.; Sun, R.; Zhao, L.; Yan, C.; Chu, H. Molecularly imprinted electrochemical sensor based on synergistic interaction of honeycomb-like Ni-MOF decorated with AgNPs and N-GQDs for ultra-sensitive detection of olaquindox in animal-origin food. Food Chem. 2023, 418, 136001–136010. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Rahman, S.; Bozal-Palabiyik, B.; Unal, D.N.; Erkmen, C.; Siddiq, M.; Shah, A.; Uslu, B. Molecularly imprinted polymers (MIPs) combined with nanomaterials as electrochemical sensing applications for environmental pollutants. Trends Environ. Anal. 2022, 36, e00176. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Li, S.; Ruan, X.; Fei, M.; Zhou, Y.; Niu, X.; Zhu, W.; Du, D.; Lin, Y. Molecularly imprinted polypyrrole nanotubes based electrochemical sensor for glyphosate detection. Biosens. Bioelectron. 2021, 191, 113434–113441. [Google Scholar] [CrossRef]
- Karthika, P.; Shanmuganathan, S.; Viswanathan, S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensor for the determination of endocrine disruptor bisphenol-A in bovine milk. Food Chem. 2021, 363, 130287–130296. [Google Scholar] [CrossRef] [PubMed]
- Devkota, L.; Nguyen, L.T.; Vu, T.T.; Piro, B. Electrochemical determination of tetracycline using AuNP-coated molecularly imprinted overoxidized polypyrrole sensing interface. Electrochim. Acta 2018, 270, 535–542. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, F.; Zeng, B. Fabrication of surface molecularly imprinted electrochemical sensor for the sensitive quantification of chlortetracycline with ionic liquid and MWCNT improving performance. Talanta 2022, 239, 123130–123138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, L.; Luo, Z.; Tang, H. Fabrication of molecular imprinted polymer sensor for chlortetracycline based on controlled electrochemical reduction of graphene oxide. Sens. Actuators B-Chem. 2013, 185, 438–444. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef]
- Wu, S.; Feng, Y.; Wu, K.; Jiang, W.; Xue, Z.; Xiong, D.; Chen, L.; Feng, Z.; Wen, K.; Li, Z.; et al. Mxene Ti3C2 generated TiO2 nanoparticles in situ and uniformly embedded in rGO sheets as high stable anodes for potassium ion batteries. J. Alloys Compd. 2023, 930, 167414–167425. [Google Scholar] [CrossRef]
- Rhouati, A.; Berkani, M.; Vasseghian, Y.; Golzadeh, N. MXene-based electrochemical sensors for detection of environmental pollutants: A comprehensive review. Chemosphere 2022, 291 Pt 1, 132921–132930. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, R.; Zhang, Y.; Huang, G.; Jiang, B.; Xu, C.; Pan, F. The design of Co3S4@MXene heterostructure as sulfur host to promote the electrochemical kinetics for reversible magnesium-sulfur batteries. J. Magnes. Alloys 2021, 9, 78–89. [Google Scholar] [CrossRef]
- Gao, F.; Hong, W.; Yang, T.; Qiao, C.; Li, J.; Xiao, X.; Zhao, Z.; Zhang, C.; Tang, J. Expanded interlayer spacing of SnO2 QDs-Decorated MXene for highly selective luteolin detection with Ultra-Low limit of detection. J. Colloid Interface Sci. 2024, 653, 561–569. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; He, Z.; Li, X.; Xu, J.; Liu, B.; Jiang, Y. Enhanced ammonia response of Ti3C2T nanosheets supported by TiO2 nanoparticles at room temperature. Sens. Actuators B-Chem. 2019, 298, 126874–126878. [Google Scholar] [CrossRef]
- Ezhil Vilian, A.T.; Hwang, S.-K.; Bhaskaran, G.; Alhammadi, M.; Kim, S.; Tiwari, J.N.; Suk Huh, Y.; Han, Y.-K. Polypyrrole-MXene supported gold nanoparticles for the trace-level detection of nitrofurantoin. Chem. Eng. J. 2023, 454, 139980–139990. [Google Scholar] [CrossRef]
- Devi, R.K.; Ganesan, M.; Chen, T.-W.; Chen, S.-M.; Abbasi, A.M.; Ali, M.A.; Elshikh, M.S.; Yu, J.; Chuang, H.-Y.; Xu, B.; et al. MXene-interdigitated Holey-graphene oxide nanocomposite for simultaneous detection of antibiotic and anticancer drugs with ultra-high sensitivity. Chem. Eng. J. 2023, 474, 145693–145706. [Google Scholar] [CrossRef]
- Ma, X.; Tu, X.; Gao, F.; Xie, Y.; Huang, X.; Fernandez, C.; Qu, F.; Liu, G.; Lu, L.; Yu, Y. Hierarchical porous MXene/amino carbon nanotubes-based molecular imprinting sensor for highly sensitive and selective sensing of fisetin. Sens. Actuators B-Chem. 2020, 309, 127815–127824. [Google Scholar] [CrossRef]
- Yang, J.; Deng, C.; Zhong, W.; Peng, G.; Zou, J.; Lu, Y.; Gao, Y.; Li, M.; Zhang, S.; Lu, L. Electrochemical activation of oxygen vacancy-rich TiO2@MXene as high-performance electrochemical sensing platform for detecting imidacloprid in fruits and vegetables. Microchim. Acta 2023, 190, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Yang, S.; Shan, J. A novel electrochemical sensor based on TiO2–Ti3C2TX/CTAB/chitosan composite for the detection of nitrite. Electrochim. Acta 2020, 359, 136938–136947. [Google Scholar] [CrossRef]
- Zhong, W.; Zou, J.; Yu, Q.; Gao, Y.; Qu, F.; Liu, S.; Zhou, H.; Lu, L. Ultrasensitive indirect electrochemical sensing of thiabendazole in fruit and water by the anodic stripping voltammetry of Cu2+ with hierarchical Ti3C2Tx-TiO2 for signal amplification. Food Chem. 2023, 402, 134379–134386. [Google Scholar] [CrossRef]
- Liu, N.; Yu, L.; Liu, B.; Yu, F.; Li, L.; Xiao, Y.; Yang, J.; Ma, J. Ti3C2-MXene Partially Derived Hierarchical 1D/2D TiO2/Ti3C2 Heterostructure Electrode for High-Performance Capacitive Deionization. Adv. Sci. 2023, 10, e2204041. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Nawaz, M.; Miran, W.; Jang, J.; Moztahida, M.; Mahmoud, K.A.; Lee, D.S. Heterostructural TiO2/Ti3C2Tx (MXene) for photocatalytic degradation of antiepileptic drug carbamazepine. Chem. Eng. J. 2018, 349, 748–755. [Google Scholar] [CrossRef]
- Wang, S.X.; Ma, R.R.; Mazzu, Y.Z.; Zhang, J.W.; Li, W.; Tan, L.; Zhou, L.D.; Xia, Z.N.; Zhang, Q.H.; Yuan, C.S. Specific adsorption of tetracycline from milk by using biocompatible magnetic molecular imprinting material and evaluation by ECD. Food Chem. 2020, 326, 126969–126976. [Google Scholar] [CrossRef]
- Lian, W.; Huang, J.; Yu, J.; Zhang, X.; Lin, Q.; He, X.; Xing, X.; Liu, S. A molecularly imprinted sensor based on β-cyclodextrin incorporated multiwalled carbon nanotube and gold nanoparticles-polyamide amine dendrimer nanocomposites combining with water-soluble chitosan derivative for the detection of chlortetracycline. Food Control 2012, 26, 620–627. [Google Scholar] [CrossRef]
- Masawat, P.; Slater, J.M. The determination of tetracycline residues in food using a disposable screen-printed gold electrode (SPGE). Sens. Actuators B-Chem. 2007, 124, 127–132. [Google Scholar] [CrossRef]
- Wangfuengkanagul, N.; Siangproh, W.; Chailapakul, O. A flow injection method for the analysis of tetracycline antibiotics in pharmaceutical formulations using electrochemical detection at anodized boron-doped diamond thin film electrode. Talanta 2004, 64, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- GB/T 5009.116-2003; Determination of Oxytetracyline, Tetrayline and Chlortetracycline Residues in Meat (HPLC). Ministry of Health of the People’s Republic of China; Standardization Administration of the People’s Republic of China: Beijing, China, 2003.






| Electrode | Detection Technology | Linear Range (μM) | LOD (μM) | References |
|---|---|---|---|---|
| IL1-SMIP/MWCNT-IL/GCE | LSV | 0.4–55 | 0.08 | [23] |
| BMMIPs/GCE | DPV | 0.052–1044 | 0.052 | [39] |
| RGO-MIP/GCE | DPV | 10–500 | - | [24] |
| CD-MWCNTs/PAMAM-Au/MIPs/GE | i-t | 0.09–50 | 0.0495 | [40] |
| SPGE | CV | 5–50 | 0.58 | [41] |
| BDDE | CV | 500–50,000 | 0.01 | [42] |
| MIP/TiO2@Ti3C2Tx/GCE | DPV | 0.00006–1 | 0.000027 | This work |
| Sample | Number | Spiked Concentration (μM) | Determined by Target Sensor (μM) | Determined by HPLC (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Chicken | 1 | 0.00 | ND | ND | - | - |
| 2 | 0.20 | 0.197 | 0.184 | 98.7 | 3.91 | |
| 3 | 0.40 | 0.396 | 0.364 | 99 | 1.72 | |
| 4 | 0.80 | 0.783 | 0.688 | 97.9 | 2.51 | |
| Milk | 1 | 0.00 | ND | ND | - | - |
| 2 | 0.20 | 0.205 | 0.188 | 102.4 | 3.22 | |
| 3 | 0.40 | 0.394 | 0.364 | 98.5 | 2.95 | |
| 4 | 0.80 | 0.803 | 0.656 | 100.4 | 3.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Liu, J.; Huang, H.; Deng, C.; Lu, L.; Wang, L.; Wang, X. A Molecularly Imprinted Electrochemical Sensor Based on TiO2@Ti3C2Tx for Highly Sensitive and Selective Detection of Chlortetracycline. Molecules 2023, 28, 7475. https://doi.org/10.3390/molecules28227475
Deng L, Liu J, Huang H, Deng C, Lu L, Wang L, Wang X. A Molecularly Imprinted Electrochemical Sensor Based on TiO2@Ti3C2Tx for Highly Sensitive and Selective Detection of Chlortetracycline. Molecules. 2023; 28(22):7475. https://doi.org/10.3390/molecules28227475
Chicago/Turabian StyleDeng, Linbo, Jiawei Liu, Haiyan Huang, Changxi Deng, Limin Lu, Linyu Wang, and Xiaoqiang Wang. 2023. "A Molecularly Imprinted Electrochemical Sensor Based on TiO2@Ti3C2Tx for Highly Sensitive and Selective Detection of Chlortetracycline" Molecules 28, no. 22: 7475. https://doi.org/10.3390/molecules28227475