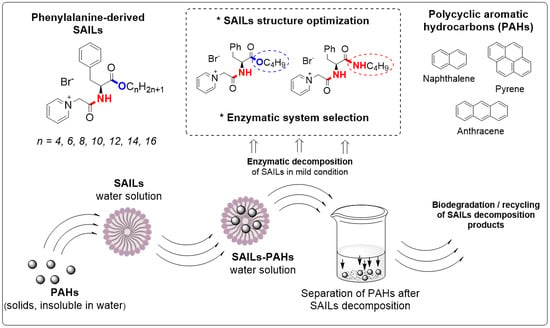
Sustainable Phenylalanine-Derived SAILs for Solubilization of Polycyclic Aromatic Hydrocarbons
Abstract
:1. Introduction
2. Results and Discussion
2.1. Study of Solubilization Capacity
2.2. Study of Enzymatic Degradation/Hydrolysis of PyPheOCn SAILs
2.3. Structural Modification of PyPheOC4 SAIL: Diamide Derivative PyPheNHC4
2.4. Study of Enzymatic Degradation/Hydrolysis of PyPheNHC4 SAIL
2.5. Concept of PyPheOCn and PyPheNHCn SAILs Application to Solubilization of Polycyclic Aromatic Hydrocarbons
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Study of Solubilization Capacity
3.2.2. Enzymatic Degradation/Hydrolysis Studies
3.2.3. NMR Analysis of Enzymatic Degradation/Hydrolysis Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef] [PubMed]
- Plechkova, N.V.; Seddon, K.R. Ionic Liquids: “Designer” Solvents for Green Chemistry. In Methods and Reagents for Green Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 103–130. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef] [PubMed]
- Pillai, P.; Maiti, M.; Mandal, A. Mini-review on Recent Advances in the Application of Surface-Active Ionic Liquids: Petroleum Industry Perspective. Energy Fuels 2022, 36, 7925–7939. [Google Scholar] [CrossRef]
- Jordan, A.; Gathergood, N. Biodegradation of ionic liquids–a critical review. Chem. Soc. Rev. 2015, 44, 8200–8237. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef]
- Suk, M.; Haiß, A.; Westphal, J.; Jordan, A.; Kellett, A.; Kapitanov, I.V.; Karpichev, Y.; Gathergood, N.; Kümmerer, K. Design rules for environmental biodegradability of phenylalanine alkyl ester linked ionic liquids. Green Chem. 2020, 22, 4498–4508. [Google Scholar] [CrossRef]
- Gu, Y.; Jérôme, F. Bio-based solvents: An emerging generation of fluids for the design of eco-efficient processes in catalysis and organic chemistry. Chem. Soc. Rev. 2013, 42, 9550–9570. [Google Scholar] [CrossRef]
- Kapitanov, I.V.; Mirgorodskaya, A.B.; Valeeva, F.G.; Gathergood, N.; Kuca, K.; Zakharova, L.Y.; Karpichev, Y. Physicochemical properties and esterolytic reactivity of oxime functionalized surfactants in pH-responsive mixed micellar system. Colloids Surf. A Physicochem. Eng. Asp. 2017, 524, 143–159. [Google Scholar] [CrossRef]
- Pandya, S.J.; Kapitanov, I.V.; Usmani, Z.; Sahu, R.; Sinha, D.; Gathergood, N.; Ghosh, K.K.; Karpichev, Y. An example of green surfactant systems based on inherently biodegradable IL-derived amphiphilic oximes. J. Mol. Liq. 2020, 305, 112857. [Google Scholar] [CrossRef]
- Banjare, M.K.; Behera, K.; Banjare, R.K.; Pandey, S.; Ghosh, K.K.; Karpichev, Y. Molecular interactions between novel synthesized biodegradable ionic liquids with antidepressant drug. Chem. Thermodyn. Therm. Anal. 2021, 3, 100012. [Google Scholar] [CrossRef]
- Pandya, S.J.; Kapitanov, I.V.; Banjare, M.K.; Behera, K.; Borovkov, V.; Ghosh, K.K.; Karpichev, Y. Mixed Oxime-Functionalized IL/16-s-16 Gemini Surfactants System: Physicochemical Study and Structural Transitions in the Presence of Promethazine as a Potential Chiral Pollutant. Chemosensors 2022, 10, 46. [Google Scholar] [CrossRef]
- Gonçalves, A.R.; Paredes, X.; Cristino, A.F.; Santos, F.J.; Queirós, C.S. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, D.; Maculewicz, J.; Stepnowski, P.; Dołżonek, J. Ionic liquids as environmental hazards–Crucial data in view of future PBT and PMT assessment. J. Hazard. Mater. 2021, 403, 123896. [Google Scholar] [CrossRef]
- Amsel, A.-K.; Olsson, O.; Kümmerer, K. Inventory of biodegradation data of ionic liquids. Chemosphere 2022, 299, 134385. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Radošević, K.; Redovniković, I.R.; Slivac, I.; Srček, V.G. Toxicity mechanisms of ionic liquids. Arch. Ind. Hyg. Toxicol. 2017, 68, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.M.; Aiello, S.W.; Buehler, B.K.; Jones, S.E.; Szymczyna, B.R.; Walker, K.A. Ionic liquid biodegradability depends on specific wastewater microbial consortia. Chemosphere 2015, 136, 160–166. [Google Scholar] [CrossRef]
- Kusumahastuti, D.K.; Sihtmäe, M.; Kapitanov, I.V.; Karpichev, Y.; Gathergood, N.; Kahru, A. Toxicity profiling of 24 l-phenylalanine derived ionic liquids based on pyridinium, imidazolium and cholinium cations and varying alkyl chains using rapid screening Vibrio fischeri bioassay. Ecotoxicol. Environ. Saf. 2019, 172, 556–565. [Google Scholar] [CrossRef]
- Pinazo, A.; Manresa, M.; Marques, A.; Bustelo, M.; Espuny, M.; Pérez, L. Amino acid–based surfactants: New antimicrobial agents. Adv. Colloid Interface Sci. 2016, 228, 17–39. [Google Scholar] [CrossRef]
- Mero, A.; Mezzetta, A.; Nowicki, J.; Łuczak, J.; Guazzelli, L. Betaine and l-carnitine ester bromides: Synthesis and comparative study of their thermal behaviour and surface activity. J. Mol. Liq. 2021, 334, 115988. [Google Scholar] [CrossRef]
- Mezzetta, A.; Łuczak, J.; Woch, J.; Chiappe, C.; Nowicki, J.; Guazzelli, L. Surface active fatty acid ILs: Influence of the hydrophobic tail and/or the imidazolium hydroxyl functionalization on aggregates formation. J. Mol. Liq. 2019, 289, 111155. [Google Scholar] [CrossRef]
- Rantamäki, A.H.; Ruokonen, S.-K.; Sklavounos, E.; Kyllönen, L.; King, A.W.T.; Wiedmer, S.K. Impact of Surface-Active Guanidinium-, Tetramethylguanidinium-, and Cholinium-Based Ionic Liquids on Vibrio Fischeri Cells and Dipalmitoylphosphatidylcholine Liposomes. Sci. Rep. 2017, 7, 46673. [Google Scholar] [CrossRef]
- Jordan, A.; Haiß, A.; Spulak, M.; Karpichev, Y.; Kümmerer, K.; Gathergood, N. Synthesis of a series of amino acid derived ionic liquids and tertiary amines: Green chemistry metrics including microbial toxicity and preliminary biodegradation data analysis. Green Chem. 2016, 18, 4374–4392. [Google Scholar] [CrossRef]
- Kapitanov, I.V.; Raba, G.; Špulák, M.; Vilu, R.; Karpichev, Y.; Gathergood, N. Design of sustainable ionic liquids based on l-phenylalanine and l-alanine dipeptides: Synthesis, toxicity and biodegradation studies. J. Mol. Liq. 2023, 374, 121285. [Google Scholar] [CrossRef]
- Haiß, A.; Jordan, A.; Westphal, J.; Logunova, E.; Gathergood, N.; Kümmerer, K. On the way to greener ionic liquids: Identification of a fully mineralizable phenylalanine-based ionic liquid. Green Chem. 2016, 18, 4361–4373. [Google Scholar] [CrossRef]
- OECD. OECD Test Guidelines for Chemicals; OECD: Paris, France, 1992. [Google Scholar]
- Friedrich, J.; Längin, A.; Kümmerer, K. Comparison of an Electrochemical and Luminescence-Based Oxygen Measuring System for Use in the Biodegradability Testing According to Closed Bottle Test (OECD 301D). Clean 2013, 41, 251–257. [Google Scholar] [CrossRef]
- Zubareva, T.M.; Anikeev, A.V.; Karpichev, E.A.; Red’ko, A.N.; Prokop’eva, T.M.; Popov, A.F. Cleavable dicationic surfactant micellar system for the decomposition of organophosphorus compounds. Theor. Exp. Chem. 2012, 47, 377–383. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.; Holmberg, K. Cleavable surfactants. Curr. Opin. Colloid Interface Sci. 2007, 12, 81–91. [Google Scholar] [CrossRef]
- Stolte, S.; Steudte, S.; Areitioaurtena, O.; Pagano, F.; Thöming, J.; Stepnowski, P.; Igartua, A. Ionic liquids as lubricants or lubrication additives: An ecotoxicity and biodegradability assessment. Chemosphere 2012, 89, 1135–1141. [Google Scholar] [CrossRef]
- Stolte, S.; Steudte, S.; Igartua, A.; Stepnowski, P. The Biodegradation of Ionic Liquids-the View from a Chemical Structure Perspective. Curr. Org. Chem. 2011, 15, 1946–1973. [Google Scholar] [CrossRef]
- Kapitanov, I.V.; Jordan, A.; Karpichev, Y.; Spulak, M.; Perez, L.; Kellett, A.; Kümmerer, K.; Gathergood, N. Synthesis, self-assembly, bacterial and fungal toxicity, and preliminary biodegradation studies of a series ofl-phenylalanine-derived surface-active ionic liquids. Green Chem. 2019, 21, 1777–1794. [Google Scholar] [CrossRef]
- Coleman, D.; Gathergood, N. Biodegradation studies of ionic liquids. Chem. Soc. Rev. 2010, 39, 600–637. [Google Scholar] [CrossRef]
- Gathergood, N.; Garcia, M.T.; Scammells, P.J. Biodegradable ionic liquids: Part I. Concept, preliminary targets and evaluation. Green Chem. 2004, 6, 166–175. [Google Scholar] [CrossRef]
- Garcia, M.T.; Gathergood, N.; Scammells, P.J. Biodegradable ionic liquids: Part II. Effect of the anion and toxicology. Green Chem. 2005, 7, 9–14. [Google Scholar] [CrossRef]
- Gathergood, N.; Scammells, P.J.; Garcia, M.T. Biodegradable ionic liquids: Part III. The first readily biodegradable ionic liquids. Green Chem. 2006, 8, 156–160. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, P.; Sharma, J.; Thapa, S.D.; Gupta, A.; Rajak, R.; Baruah, B.; Prakash, A.; Ranjan, R.K. Characterization of Polycyclic Aromatic Hydrocarbons (PAHs) associated with fine aerosols in ambient atmosphere of high-altitude urban environment in Sikkim Himalaya. Sci. Total Environ. 2023, 870, 161987. [Google Scholar] [CrossRef]
- Serdyuk, A.A.; Mirgorodskaya, A.B.; Kapitanov, I.V.; Gathergood, N.; Zakharova, L.Y.; Sinyashin, O.G.; Karpichev, Y. Effect of structure of polycyclic aromatic substrates on solubilization capacity and size of cationic monomeric and gemini 14-s-14 surfactant aggregates. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 613–622. [Google Scholar] [CrossRef]
- Seitkalieva, M.M.; Kashin, A.S.; Egorova, K.S.; Ananikov, V.P. Ionic Liquids As Tunable Toxicity Storage Media for Sustainable Chemical Waste Management. ACS Sustain. Chem. Eng. 2018, 6, 719–726. [Google Scholar] [CrossRef]
- Holmberg, K. (Ed.) Handbook of Applied Surface and Colloid Chemistry; John Wiley & Sons: Chichester, UK, 2002. [Google Scholar]
- Zakharova, L.Y.; Serdyuk, A.A.; Mirgorodskaya, A.B.; Kapitanov, I.V.; Gainanova, G.A.; Karpichev, Y.; Gavrilova, E.L.; Sinyashin, O.G. Amino Acid-Functionalized Calix [4] Resorcinarene Solubilization by Mono- and Dicationic Surfactants. J. Surfactants Deterg. 2016, 19, 493–499. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Karpichev, Y.; Zakharova, L.Y.; Yackevich, E.I.; Kapitanov, I.V.; Lukashenko, S.S.; Popov, A.F.; Konovalov, A.I. Aggregation behavior and interface properties of mixed surfactant systems gemini 14-s-14/CTABr. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 425–432. [Google Scholar] [CrossRef]
- Konopka, A.; Zakharova, T.; Oliver, L.; Turco, R. Microbial biodegradation of organic wastes containing surfactants in a continuous-flow reactor. J. Ind. Microbiol. Biotechnol. 1997, 18, 235–240. [Google Scholar] [CrossRef]
- Sudheer, S.; Raba, G.; Kapitanov, I.; Karpichev, Y.; Gupta, V.K.; Vilu, R.; Gathergood, N. A Greener Approach to Hydrolyse Ionic Liquids. Basic Clin. Pharmacol. Toxicol. 2018, 124, 21. [Google Scholar]
- Ray, S.S. Environmentally friendly polymer matrices for composites. In Environmentally Friendly Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2013; pp. 25–40. [Google Scholar] [CrossRef]
- Neumann, J.; Steudte, S.; Cho, C.-W.; Thöming, J.; Stolte, S. Biodegradability of 27 pyrrolidinium, morpholinium, piperidinium, imidazolium and pyridinium ionic liquid cations under aerobic conditions. Green Chem. 2014, 16, 2174–2184. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, S. Metalloenzyme nitrile hydratase: Structure, regulation, and application to biotechnology. Nat. Biotechnol. 1998, 16, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Mascharak, P.K. Structural and functional models of nitrile hydratase. Coord. Chem. Rev. 2002, 225, 201–214. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Salvesen, G. (Eds.) Handbook of Proteolytic Enzymes, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Tomihata, K.; Ikada, Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 1997, 18, 567–575. [Google Scholar] [CrossRef]
- Klebe, G. Inhibitors of Hydrolases with an Acyl–Enzyme Intermediate. In Drug Design; Springer: Berlin/Heidelberg, Germany, 2013; pp. 493–532. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- López-Otín, C.; Bond, J.S. Proteases: Multifunctional Enzymes in Life and Disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, R.J.; Rawn, J.D. Principles of Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Areekijseree, M.; Engkagul, A.; Kovitvadhi, U.; Thongpan, A.; Mingmuang, M.; Pakkong, P.; Rungruangsak-Torrissen, K. Temperature and pH characteristics of amylase and proteinase of adult freshwater pearl mussel, Hyriopsis (Hyriopsis) bialatus Simpson 1900. Aquaculture 2004, 234, 575–587. [Google Scholar] [CrossRef]
- Azevedo, H.S.; Reis, R.L. Understanding the Enzymatic Degradation of Biodegradable Polymers and Strategies to Control Their Degradation Rate. In Biodegradable Systems in Tissue Engineering and Regenerative Medicine; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Bender, M.L.; Ginger, R.D.; Unik, J.P. Activation Energies of the Hydrolysis of Esters and Amides Involving Carbonyl Oxygen Exchange 1. J. Am. Chem. Soc. 1958, 80, 1044–1048. [Google Scholar] [CrossRef]
- Jencks, W.P.; Carriuolo, J. Reactivity of Nucleophilic Reagents toward Esters. J. Am. Chem. Soc. 1960, 82, 1778–1786. [Google Scholar] [CrossRef]














| PyPheOCn Chain Length (n) | cmc, mM | β | Solubilization Capacity (S) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface Tension | Conductivity | Solubilization | |||||||||
| Nap | Ant | Pyr | Nap | Ant | Pyr | Nap | Ant | Pyr | |||
| 4 | 57 | 89 | 80 | 90 | 80 | 21.4 | 5.6 | 90.4 | 0.067 | 0.00037 | 0.0014 |
| 6 | 11 | 19.9 | 25 | 23 | 26 | 32.7 | 9 | 251.9 | 0.102 | 0.00060 | 0.0040 |
| 8 | 2.25 | 4.9 | 5.2 | 3.7 | 4.9 | 36.9 | 11.7 | 366.6 | 0.115 | 0.00077 | 0.0058 |
| 10 | 0.65 | 1.6 | 1.6 | 1 | 1.4 | 48.1 | 27.9 | 503 | 0.150 | 0.00185 | 0.0080 |
| 12 | 0.19 | 0.5 | 0.2 | 0.1 | 0.3 | 67.1 | 31.3 | 745.7 | 0.210 | 0.00207 | 0.0119 |
| 14 | 0.056 | 0.18 | 0.1 | 0.07 | 0.08 | 84.9 | 47.4 | 905.2 | 0.265 | 0.00314 | 0.0144 |
| 16 | 0.0125 | 0.037 | 0.05 | 0.04 | 0.05 | 100.5 | 60.3 | 996 | 0.314 | 0.00399 | 0.0159 |
| CTABr | 0.9 | 1 | 1 | 1 | 0.8 | 62.5 | 43.9 | 573.6 | 0.195 | 0.00291 | 0.0091 |
| Enzyme | Source | Activity |
|---|---|---|
| A1 | Escherichia coli | NLT 850 U/g |
| A2 | Achomobacter | NLT 250 U/g |
| E1 | Pichia sp. | NLT 10,000 PLU/g |
| E2 | Pichia sp. | NLT 10,000 PLU/g |
| P1 | Bacillus sp. | 400 ELU/g |
| P2 | Bacillus sp. | 750 ELU/g |
| P3 | Bacillus sp. | 100 ELU/g |
| P4 | Bacillus sp. | 275 ELU/g |
| P5 | Mucor miehei | 8 ELU/g |
| P6 | Bacillus licheniformis | 400 ELU/g |
| P7 | Bacillus amyloliquefaciens | 15 ELU/g |
| P8 | Geobacillus sp. | 5 ELU/g |
| P9 | Trichoderma reesei | 5 ELU/g |
| P10 | Bacillus subtilis | 225 ELU/g |
| P11 | Bacillus subtilis | 400 ELU/g |
| P12 | Aspergillus oryzae var. | 5 ELU/g |
| P13 | Aspergillus oryzae | 65 ELU/g |
| P14 | Bacillus subtilis | 150 ELU/g |
| P15 | Aspergillus niger | 5 ELU/g |
| P16 | Bacillus subtilis | 10 ELU/g |
| P17 | Bacillus subtilis | 175 ELU/g |
| P18 | Carica papaya | 5 ELU/g |
| P19 | Pineapple stem | 5 ELU/g |
| P20 | Fig tree latex | 5 ELU/g |
| Test | ILs Tested | IL Test Conc. (% w/v) | pH | Enzymes Tested | Enzyme Test Conc. (g) | Incubating Temperature (°C) | Incubation Speed (rpm) | Incubation Time (Days) |
|---|---|---|---|---|---|---|---|---|
| 1 | PyPheOC4 PyPheNHC4 | 2 | 5.5 | Amidase 1 and 2 | 0.2 | 40 | 170 | 3 days |
| 2 | PyPheOC4 PyPheNHC4 | 1 | 5.5 | Amidase 1 and 2 | 0.2 | 40 | 170 | 3 days |
| 3 | PyPheOC4 PyPheNHC4 | 1 | 5.5 | Amidase 1 and 2 Protease 1–20 | 0.1 | 50 | 100 | 7 days |
| 4 | PyPheOC4 PyPheNHC4 | 0.5 | 6.5 | Amidase 1 and 2 Protease (P1-P4, P6, P10, P11, P13, P14, P17) | 0.1 | 50 | 100 | 7 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapitanov, I.V.; Sudheer, S.M.; Yadav, T.; Ghosh, K.K.; Gathergood, N.; Gupta, V.K.; Karpichev, Y. Sustainable Phenylalanine-Derived SAILs for Solubilization of Polycyclic Aromatic Hydrocarbons. Molecules 2023, 28, 4185. https://doi.org/10.3390/molecules28104185
Kapitanov IV, Sudheer SM, Yadav T, Ghosh KK, Gathergood N, Gupta VK, Karpichev Y. Sustainable Phenylalanine-Derived SAILs for Solubilization of Polycyclic Aromatic Hydrocarbons. Molecules. 2023; 28(10):4185. https://doi.org/10.3390/molecules28104185
Chicago/Turabian StyleKapitanov, Illia V., Surya M. Sudheer, Toshikee Yadav, Kallol K. Ghosh, Nicholas Gathergood, Vijai K. Gupta, and Yevgen Karpichev. 2023. "Sustainable Phenylalanine-Derived SAILs for Solubilization of Polycyclic Aromatic Hydrocarbons" Molecules 28, no. 10: 4185. https://doi.org/10.3390/molecules28104185