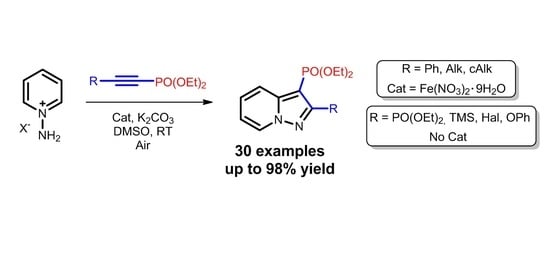
Oxidative [3+2]Cycloaddition of Alkynylphosphonates with Heterocyclic N-Imines: Synthesis of Pyrazolo[1,5-a]Pyridine-3-phosphonates
Abstract
:1. Introduction
2. Results


3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rolan, P.; Hutchinson, M.; Johnson, K. Ibudilast: A Review of Its Pharmacology, Efficacy and Safety in Respiratory and Neurological Disease. Expert Opin. Pharmacother. 2009, 10, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Ledeboer, A.; Hutchinson, M.R.; Watkins, L.R.; Johnson, K.W. Ibudilast (AV-411): A New Class Therapeutic Candidate for Neuropathic Pain and Opioid Withdrawal Syndromes. Expert Opin. Investig. Drugs 2007, 16, 935–950. [Google Scholar] [CrossRef]
- Fox, R.J.; Coffey, C.S.; Conwit, R.; Cudkowicz, M.E.; Gleason, T.; Goodman, A.; Klawiter, E.C.; Matsuda, K.; McGovern, M.; Naismith, R.T.; et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N. Engl. J. Med. 2018, 379, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.D.; Gyang, T.; Smith, A.D. Ibudilast for the Treatment of Multiple Sclerosis. Expert Opin. Investig. Drugs 2016, 25, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Nogami, M.; Iwatani, M.; Imaeda, T.; Ito, M.; Tanaka, T.; Tawada, M.; Endo, S.; Cary, D.R.; Ohori, M.; et al. Identification of a Selective DDX3X Inhibitor with Newly Developed Quantitative High-Throughput RNA Helicase Assays. Biochem. Biophys. Res. Commun. 2020, 523, 795–801. [Google Scholar] [CrossRef]
- Calbet, M.; Ramis, I.; Calama, E.; Carreño, C.; Paris, S.; Maldonado, M.; Orellana, A.; Calaf, E.; Pauta, M.; De Alba, J.; et al. Novel Inhaled Pan-JAK Inhibitor, LAS194046, Reduces Allergen-Induced Airway Inflammation, Late Asthmatic Response, and PSTAT Activation in Brown Norway Rats. J. Pharmacol. Exp. Ther. 2019, 370, 137–147. [Google Scholar] [CrossRef]
- O’Malley, D.P.; Ahuja, V.; Fink, B.; Cao, C.; Wang, C.; Swanson, J.; Wee, S.; Gavai, A.V.; Tokarski, J.; Critton, D.; et al. Discovery of Pyridazinone and Pyrazolo[1,5-a]Pyridine Inhibitors of C-Terminal Src Kinase. ACS Med. Chem. Lett. 2019, 10, 1486–1491. [Google Scholar] [CrossRef]
- Sainas, S.; Pippione, A.C.; Lupino, E.; Giorgis, M.; Circosta, P.; Gaidano, V.; Goyal, P.; Bonanni, D.; Rolando, B.; Cignetti, A.; et al. Targeting Myeloid Differentiation Using Potent 2-Hydroxypyrazolo[1,5-a]Pyridine Scaffold-Based Human Dihydroorotate Dehydrogenase Inhibitors. J. Med. Chem. 2018, 61, 6034–6055. [Google Scholar] [CrossRef]
- Kendall, J.D.; Giddens, A.C.; Tsang, K.Y.; Marshall, E.S.; Lill, C.L.; Lee, W.-J.; Kolekar, S.; Chao, M.; Malik, A.; Yu, S.; et al. Novel Pyrazolo[1,5-a]Pyridines with Improved Aqueous Solubility as P110α-Selective PI3 Kinase Inhibitors. Bioorganic Med. Chem. Lett. 2017, 27, 187–190. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chu, J.-H.; Li, C.-W.; Hwang, L.-C.; Wu, M.-J. Palladium-Catalyzed Regioselective Arylation of Pyrazolo[1,5-a]Pyridines via C–H Activation and Synthetic Applications on P38 Kinase Inhibitors. Organometallics 2016, 35, 288–300. [Google Scholar] [CrossRef]
- Lechtenberg, B.C.; Mace, P.D.; Sessions, E.H.; Williamson, R.; Stalder, R.; Wallez, Y.; Roth, G.P.; Riedl, S.J.; Pasquale, E.B. Structure-Guided Strategy for the Development of Potent Bivalent ERK Inhibitors. ACS Med. Chem. Lett. 2017, 8, 726–731. [Google Scholar] [CrossRef]
- Nirogi, R.; Mohammed, A.R.; Shinde, A.K.; Gagginapally, S.R.; Kancharla, D.M.; Middekadi, V.R.; Bogaraju, N.; Ravella, S.R.; Singh, P.; Birangal, S.R.; et al. Synthesis, Structure–Activity Relationships, and Preclinical Evaluation of Heteroaromatic Amides and 1,3,4-Oxadiazole Derivatives as 5-HT4 Receptor Partial Agonists. J. Med. Chem. 2018, 61, 4993–5008. [Google Scholar] [CrossRef] [PubMed]
- Umei, K.; Nishigaya, Y.; Kondo, A.; Tatani, K.; Tanaka, N.; Kohno, Y.; Seto, S. Novel Pyrazolo[1,5-a]Pyridines as Orally Active EP 1 Receptor Antagonists: Synthesis, Structure-Activity Relationship Studies, and Biological Evaluation. Bioorganic Med. Chem. 2017, 25, 2635–2642. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Williams, Z.; Hards, K.; Tang, J.; Cheung, C.-Y.; Aung, H.L.; Wang, B.; Liu, Z.; Hu, X.; Lenaerts, A.; et al. Pyrazolo[1,5-a]Pyridine Inhibitor of the Respiratory Cytochrome Bcc Complex for the Treatment of Drug-Resistant Tuberculosis. ACS Infect. Dis. 2019, 5, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Large, J.M.; Birchall, K.; Bouloc, N.S.; Merritt, A.T.; Smiljanic-Hurley, E.; Tsagris, D.J.; Wheldon, M.C.; Ansell, K.H.; Coombs, P.J.; Kettleborough, C.A.; et al. Potent Inhibitors of Malarial P. Falciparum Protein Kinase G: Improving the Cell Activity of a Series of Imidazopyridines. Bioorganic Med. Chem. Lett. 2019, 29, 509–514. [Google Scholar] [CrossRef]
- Kendall, J.D. Synthesis and Reactions of Pyrazolo[1,5-a]Pyridines and Related Heterocycles. Curr. Org. Chem. 2011, 15, 2481–2518. [Google Scholar] [CrossRef]
- Mohan, D.C.; Ravi, C.; Rao, S.N.; Adimurthy, S. Copper-Mediated Synthesis of Pyrazolo[1,5-a]Pyridines through Oxidative Linkage of C–C/N–N Bonds. Org. Biomol. Chem. 2015, 13, 3556–3560. [Google Scholar] [CrossRef]
- Ravi, C.; Samanta, S.; Mohan, D.; Reddy, N.; Adimurthy, S. Synthesis of Functionalized Pyrazolo[1,5-a]Pyridines: [3+2] Cycloaddition of N-Aminopyridines and α,β-Unsaturated Carbonyl Compounds/Alkenes at Room Temperature. Synthesis 2017, 49, 2513–2522. [Google Scholar] [CrossRef] [Green Version]
- Ravi, C.; Chandra Mohan, D.; Naresh Kumar Reddy, N.; Adimurthy, S. Substrate Selective Synthesis of Pyrazolo[1,5-a]Pyridines through [3+2] Cycloaddition of N-Aminopyridines and β-Nitro Styrenes. RSC Adv. 2015, 5, 42961–42964. [Google Scholar] [CrossRef]
- Motornov, V.A.; Tabolin, A.A.; Nelyubina, Y.V.; Nenajdenko, V.G.; Ioffe, S.L. Copper-Mediated Oxidative [3+2]-Annulation of Nitroalkenes and Pyridinium Imines: Efficient Synthesis of 3-Fluoro- and 3-Nitro-Pyrazolo[1,5-a]Pyridines. Org. Biomol. Chem. 2020, 18, 1436–1448. [Google Scholar] [CrossRef]
- Rodriguez, J.B.; Gallo-Rodriguez, C. The Role of the Phosphorus Atom in Drug Design. ChemMedChem 2018, 14, 190–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demmer, C.S.; Krogsgaard-Larsen, N.; Bunch, L. Review on Modern Advances of Chemical Methods for the Introduction of a Phosphonic Acid Group. Chem. Rev. 2011, 111, 7981–8006. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Jia, J.; Xie, Y.-F.; Feng, L.; Xu, H.-Q.; Meng, S.; Zhao, G.-L.; Xu, W.-R.; Ge, Y.-Q. Synthesis of Nitrogen Bridgehead Heterocycles with Phosphonates via a Novel Tandem Process. Heterocycles 2013, 87, 815. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, H.; Zhao, X. Selenium-π-Acid Catalyzed Oxidative Functionalization of Alkynes: Facile Access to Ynones and Multisubstituted Oxazoles. ACS Catal. 2018, 8, 6745–6750. [Google Scholar] [CrossRef]
- Huang, Q.; He, D.; Han, J.; Chen, J.; He, W.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. [3+2] Cycloaddition of N-Aminopyridines and Perfluoroalkynylphosphonates: Facile Synthesis of Perfluoroalkylated Pyrazolo[1,5-a]Pyridines Containing a Phosphonate Moiety. Synthesis 2018, 50, 3731–3737. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Seyferth, D.; Paetsch, J. Diels-Alder Reaction in Organometallic Chemistry. V. Tetramethyl Acetylenediphosphonate and Dimethyl Chloroacetylenephosphonate and Their Reactions with Cyclopentadiene, 1,3-Cyclohexadiene, and Diazomethane. J. Org. Chem. 1969, 34, 1483–1484. [Google Scholar] [CrossRef]
- Tverdomed, S.N.; Röschenthaler, G.-V.; Kalinovich, N.; Lork, E.; Dogadina, A.V.; Ionin, B.I. New α-Substituted Alkylbenzene- and Dialkylbenzene-1,2-Diphosphonates: Side-Chain Metalation of Tetraethyl 4-Methyl- and 4,5-Dimethylbenzene-1,2-Diphosphonates. Tetrahedron 2008, 64, 5306–5313. [Google Scholar] [CrossRef]
- Mahajna, M.; Quistad, G.B.; Casida, J.E. Retro-Diels−Alder Reaction: Possible Involvement in the Metabolic Activation of 7-Oxabicyclo[2.2.1]Hepta-2(3),5(6)-Diene-2,3-Dicarboxylates and a Phosphonate Analog. Chem. Res. Toxicol. 1996, 9, 241–246. [Google Scholar] [CrossRef]
- Selmani, S.; Schipper, D.J. Orientation Control of Molecularly Functionalized Surfaces Applied to the Simultaneous Alignment and Sorting of Carbon Nanotubes. Angew. Chem. 2018, 130, 2423–2427. [Google Scholar] [CrossRef]
- Kyba, E.P.; Rines, S.P.; Owens, P.W.; Chou, S.-S.P. A Novel Synthesis of 1,2-Diphosphorylbenzenes. Tetrahedron Lett. 1981, 22, 1875–1878. [Google Scholar] [CrossRef]
- Ziegler, T.; Layh, M.; Effenberger, F. Darstellung Hochsubstituierter Aromaten Über Diels-Alder-Reaktionen Mit 2H-Pyran-2-onen. Chem. Ber. 1987, 120, 1347–1355. [Google Scholar] [CrossRef] [Green Version]
- Artyushin, O.I.; Matveeva, E.V.; Bushmarinov, I.S.; Odinets, I.L. Water as a Promoting Media for 1,3-Dipolar Cycloaddition of Phosphorylated Azides to Internal Alkynes. Arkivoc 2012, 2012, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Vereshchagina, Y.A.; Alimova, A.Z.; Sharova, E.V.; Artyushin, O.I.; Chachkov, D.V.; Ishmaeva, E.A. Polarity and Structure of Diphosphorus-Substituted Isoxazole and 1,2,3-Triazole. Russ. J. Org. Chem. 2013, 49, 1369–1372. [Google Scholar] [CrossRef]
- Mukai, S.; Flematti, G.R.; Byrne, L.T.; Besant, P.G.; Attwood, P.V.; Piggott, M.J. Stable Triazolylphosphonate Analogues of Phosphohistidine. Amino Acids 2012, 43, 857–874. [Google Scholar] [CrossRef]
- Lukáč, M.; Hocková, D.; Keough, D.T.; Guddat, L.W.; Janeba, Z. Novel Nucleotide Analogues Bearing (1H-1,2,3-Triazol-4-Yl)Phosphonic Acid Moiety as Inhibitors of Plasmodium and Human 6-Oxopurine Phosphoribosyltransferases. Tetrahedron 2017, 73, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Matoba, K.; Yonemoto, H.; Fukui, M.; Yamazaki, T. Structural modification of bioactive compounds. II. Syntheses of aminophosphonoic acids. Chem. Pharm. Bull. 1984, 32, 3918–3925. [Google Scholar] [CrossRef] [Green Version]
- Heimgartner, H.; Mlostoń, G.; Pipiak, P. [3+2] Cycloadditions of N-Protected ‘(S)-Diazoproline’ with Selected Acetylenes. Heterocycles 2017, 95, 223. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, M.K.; Mlostoń, G.; Obijalska, E.; Heimgartner, H. Application of Diethyl Ethynephosphonate for the Synthesis of 3-Phosphonylated β-Lactams via Kinugasa Reaction. Arkivoc 2016, 2017, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zhang, Y.; Li, P.; Bi, W.; Chen, X.; Zhao, Y. Synthesis of Novel Phosphorylated Chrysin Derivatives by 1,3-Dipolar Cycloaddition Reaction. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 1–8. [Google Scholar] [CrossRef]
- Song, W.; Zheng, N.; Li, M.; Ullah, K.; Zheng, Y. Rhodium(I)-Catalyzed Azide-Alkyne Cycloaddition (RhAAC) of Internal Alkynylphosphonates with High Regioselectivities under Mild Conditions. Adv. Synth. Catal. 2018, 360, 2429–2434. [Google Scholar] [CrossRef]
- Perez, V.; Fadel, A.; Rabasso, N. Synthesis of N-Sulfonyl Ynamido-Phosphonates: Valuable Partners for Cycloadditions. Synthesis 2017, 49, 4035–4044. [Google Scholar] [CrossRef]
- Feng, Q.; Huang, H.; Sun, J. Ru-Catalyzed [3+2] Cycloaddition of Nitrile Oxides and Electron-Rich Alkynes with Reversed Regioselectivity. Org. Lett. 2021, 23, 2431–2436. [Google Scholar] [CrossRef]
- Xiang, J.; Yi, N.; Wang, R.; Lu, L.; Zou, H.; Pan, Y.; He, W. Synthesis of β-Ketophosphonates via AgNO3-Catalyzed Hydration of Alkynylphosphonates: A Rate-Enhancement Effect of Methanol. Tetrahedron 2015, 71, 694–699. [Google Scholar] [CrossRef]
- Bian, Q.; Wu, C.; Yuan, J.; Shi, Z.; Ding, T.; Huang, Y.; Xu, H.; Xu, Y. Iron Nitrate-Mediated Selective Synthesis of 3-Acyl-1,2,4-Oxadiazoles from Alkynes and Nitriles: The Dual Roles of Iron Nitrate. J. Org. Chem. 2020, 85, 4058–4066. [Google Scholar] [CrossRef]
- Lai, Z.; Li, Z.; Liu, Y.; Yang, P.; Fang, X.; Zhang, W.; Liu, B.; Chang, H.; Xu, H.; Xu, Y. Iron-Mediated Synthesis of Isoxazoles from Alkynes: Using Iron(III) Nitrate as a Nitration and Cyclization Reagent. J. Org. Chem. 2018, 83, 145–153. [Google Scholar] [CrossRef]
- The Crystallographic Data Was Deposited at the Cambridge Crystallographic Data Center, CCDC 200118. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 28 October 2022).
- Tsuchiya, T.; Kurita, J.; Snieckus, V. General Photochemical Synthesis of 1H-1,2-Benzodiazepines from N-Iminoquinolinium Ylide Dimers. J. Org. Chem. 1977, 42, 1856–1862. [Google Scholar] [CrossRef]
- Huisgen, R.; Grashey, R.; Krischke, R. 1,3-Dipolare Cycloadditionen, 84. Additionen mit Chinolinium-, Isochinolinium- und Phenanthridinium-N-imid2). Justus Liebigs Ann. Chem. 1977, 1977, 506–527. [Google Scholar] [CrossRef]
- The Crystallographic Data Was Deposited at the Cambridge Crystallographic Data Center, CCDC 2122366. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 28 October 2022).
- Supranovich, V.I.; Vorob’ev, A.Y.; Borodkin, G.I.; Gatilov, Y.V.; Shubin, V.G. Study on Selectivity in the Reaction of 2-Substituted Pyridinium- N-Imines with Dimethyl Acetylenedicarboxylate. Tetrahedron Lett. 2016, 57, 1093–1096. [Google Scholar] [CrossRef]
- Tamura, Y.; Minamikawa, J.; Ikeda, M. O-Mesitylenesulfonylhydroxylamine and Related Compounds-Powerful Aminating Reagents. Synthesis 1977, 1–17. [Google Scholar] [CrossRef]
- Vorob’ev, A.Y.; Supranovich, V.I.; Borodkin, G.I.; Shubin, V.G. New approach toward the synthesis of deuterated pyrazolo[1,5-a]pyridines and 1,2,4-triazolo[1,5-a]pyridines. Beilstein J. Org. Chem. 2017, 13, 800–805. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Chen, X.; Yuan, J.; Qu, L.; Li, X.; Wang, F.; Ding, X.; Zhao, Y. CuSO4·5H2O-catalyzed alkynylphosphonates formation—An efficient coupling reaction of terminal alkynes with H-phosphonates. Can. J. Chem. 2012, 90, 747–752. [Google Scholar] [CrossRef]
- Egorova, A.V.; Viktorov, N.B.; Starova, G.L.; Svintsitskaya, N.I.; Garabadziu, A.V.; Dogadina, A.V. BF3·Et2O catalyzed intramolecular cyclization of diethyl 2-(dialkoxyphosphorylethynyl)-2-arylaminomalonates to 3-phosphonylated indoles. Tetrahedron Lett. 2017, 58, 2997–3001. [Google Scholar] [CrossRef]
- Kruglov, S.V.; Ignat’ev, V.M.; Ionin, B.I.; Petrov, A.A. Synthesis of Symmetrical and Mixed Diphosphonic Esters. J. General Chem. USSR 1973, 43, 1470–1480. [Google Scholar]
- Oakdale, J.S.; Sit, R.K.; Fokin, V.V. Ruthenium-Catalyzed Cycloadditions of 1-Haloalkynes with Nitrile Oxides and Organic Azides: Synthesis of 4-Haloisoxazoles and 5-Halotriazoles. Chem.-Eur. J. 2014, 20, 11101–11110. [Google Scholar] [CrossRef]
- Marian, A.; Maas, G. Diethyl (iodoethynyl)phosphonate and (iodoethynyl)diphenylphosphane oxide: Crystal structures and some cycloaddition reactions. Z. Nat. B 2020, 75, 529–536. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SADABS 1996, Program for Empirical Adsorption Correction. Available online: https://www.scienceopen.com (accessed on 28 October 2022).
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. Sect. A Found. Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]




| Entry | Solvent/Additive 1 | Conversion of 2a, % 2 |
|---|---|---|
| 1 | CH3CN/– | 63 |
| 2 | CH3CN/– 3 | 100 (64 4) |
| 3 | CH3CN/AgNO3 (10 mol%) | 63 |
| 4 | CH3CN/LiCl (10 mol%) | 80 |
| 5 | CH3CN/LiCl (20 mol%) | 56 |
| 6 | CH3CN/Ni(OAc)2 (10 mol%) | 64 |
| 7 | CH3CN/Co(NO3)2 (10 mol%) | 60 |
| 8 | CH3CN/chloranil or DDQ (1 eq) | 0 |
| 9 | CH3CN/CuSO4 (10 mol%) | 0 |
| 10 | CH3CN/CuI (10 mol%) | 0 |
| 11 | CH3CN/Fe(NO3)3·9H2O (10 mol%) | 88 |
| 12 | DMF/Fe(NO3)3·9H2O (10 mol%) | 82 |
| 13 | DMSO/Fe(NO3)3·9H2O (10 mol%) | 100 (84 4) |
| 14 | DMSO/Fe(NO3)3·9H2O (20 mol%) | 100 (84 4) |
| 15 | DMSO/Fe(NO3)3·9H2O (5 mol%) | 89 |
| 16 | DMSO/Fe(NO3)3·9H2O (10 mol%), 60 °C | 67 |
| 17 | DMSO/FeCl3 | 47 |
| 18 | DMSO/FeSO4·5H2O (10 mol%) | 66 |
| 19 | DMSO/Fe(NO3)3·9H2O (10 mol%) under Ar | 15 |
| 20 | DMSO/Fe(NO3)3·9H2O (1 eq) under Ar | 100 (70 4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippov, I.; Gatilov, Y.; Sonina, A.; Vorob’ev, A. Oxidative [3+2]Cycloaddition of Alkynylphosphonates with Heterocyclic N-Imines: Synthesis of Pyrazolo[1,5-a]Pyridine-3-phosphonates. Molecules 2022, 27, 7913. https://doi.org/10.3390/molecules27227913
Philippov I, Gatilov Y, Sonina A, Vorob’ev A. Oxidative [3+2]Cycloaddition of Alkynylphosphonates with Heterocyclic N-Imines: Synthesis of Pyrazolo[1,5-a]Pyridine-3-phosphonates. Molecules. 2022; 27(22):7913. https://doi.org/10.3390/molecules27227913
Chicago/Turabian StylePhilippov, Igor, Yuriy Gatilov, Alina Sonina, and Aleksey Vorob’ev. 2022. "Oxidative [3+2]Cycloaddition of Alkynylphosphonates with Heterocyclic N-Imines: Synthesis of Pyrazolo[1,5-a]Pyridine-3-phosphonates" Molecules 27, no. 22: 7913. https://doi.org/10.3390/molecules27227913