Olfactory Stimulation Successfully Modulates the Neurochemical, Biochemical and Behavioral Phenotypes of the Visceral Pain
Abstract
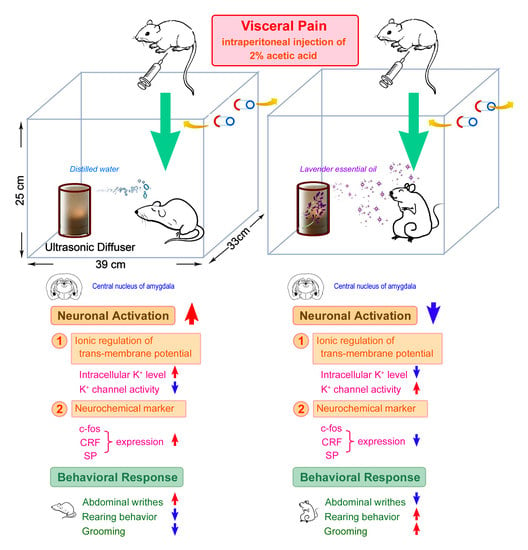
:1. Introduction
2. Results
2.1. OS Effectively Suppresses the CRF Expression in the CeA Following Visceral Pain
2.2. OS Significantly Suppresses the SP Expression in the CeA Following Visceral Pain
2.3. OS Effectively Decreases the c-fos Expression in the CeA Following Visceral Pain
2.4. OS Successfully Increases the SK Channel Protein Level in the CeA Following Visceral Pain
2.5. OS Successfully Normalizes the Intracellular K+ Level in the CeA Following Visceral Pain
2.6. OS Significantly Depresses Microglia Activation and Reduces Pro-Inflammatory Cytokine Level in the CeA Following Visceral Pain
2.7. OS Significantly Declines the Urinary NE Level Induced by Visceral Pain
2.8. OS Successfully Improves the Behavioral Responses Following Visceral Pain
3. Discussion
4. Materials and Methods
4.1. Treatment of Experimental Animals
4.2. Observation and Quantification of Behavioral Responses
4.3. Perfusion and Tissue Preparation
4.4. Corticotropin-Releasing Factor, Substance P, c-fos, and Iba1 Immunohistochemistry
4.5. TOF-SIMS Analysis
4.6. Immunobloting Assay for SK Channel Protein Level
4.7. Luminex Analysis of the Pro-Inflammatory Cytokine Levels
4.8. Biochemical Measurement of Urinary Level of Norepinephrine
4.9. Quantitative Study and Image Analysis for Neurochemical Phenotypes
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sikandar, S.; Dickenson, A.H. Visceral pain—The ins and outs, the ups and downs. Curr. Opin. Support Palliat. Care 2012, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Meerveld, B.G.; Johnson, A.C. Mechanisms of stress-induced visceral pain. J. Neurogastroenterol. Motil. 2018, 24, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Neugebauer, V. Synaptic plasticity in the amygdala in a visceral pain model in rats. Neurosci. Lett. 2004, 361, 254–257. [Google Scholar] [CrossRef]
- Simons, L.E.; Moulton, E.A.; Linnman, C.; Carpino, E.; Becerra, L.; Borsook, D. The human amygdala and pain: Evidence from neuroimaging. Hum. Brain Mapp. 2014, 35, 527–538. [Google Scholar] [CrossRef] [Green Version]
- De Berry, J.J.; Robbins, M.T.; Ness, T.J. The amygdala central nucleus is required for acute stress-induced bladder hyperalgesia in a rat visceral pain model. Brain Res. 2015, 1606, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.S.; Ku, Y.H.; Li, L.S.; Lu, Y.C.; Ding, X.; Wang, Y.G. Corticotropin releasing factor and substance P mediate the nucleus amygdaloideus centralis-nucleus ventromedialis-nucleus dorsomedialis pressor system. Brain Res. 1999, 842, 392–398. [Google Scholar] [CrossRef]
- Hwang, B.H.; Katner, J.; Iyengar, S. Corticotropin-releasing factor mRNA and substance P receptor binding in the paraventricular hypothalamic nucleus, central nucleus of the amygdala, and locus coeruleus of Sprague-Dawley rats following restraint-induced stress. J. Mol. Neurosci. 2005, 25, 239–250. [Google Scholar] [CrossRef]
- Nishii, H.; Nomura, M.; Aono, H.; Fujimoto, N.; Matsumoto, T. Up-regulation of galanin and corticotropin-releasing hormone mRNAs in the key hypothalamic and amygdaloid nuclei in a mouse model of visceral pain. Regul. Pept. 2007, 141, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Taché, Y. Corticotrophin-releasing factor 1 activation in the central amygdale and visceral hyperalgesia. Neurogastroenterol. Motil. 2015, 27, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bahia, P.K.; Suzuki, R.; Benton, D.C.; Jowett, A.J.; Chen, M.X.; Trezise, D.J.; Dickenson, A.H.; Moss, G.W.J. A functional role for small-conductance calcium-activated potassium channels in sensory pathways including nociceptive processes. J. Neurosci. 2005, 25, 3489–3498. [Google Scholar] [CrossRef]
- Thompson, J.M.; Yakhnitsa, V.; Ji, G.; Neugebauer, V. Small conductance calcium activated potassium (SK) channel dependent and independent effects of riluzole on neuropathic pain-related amygdala activity and behaviors in rats. Neuropharmacology 2018, 138, 219–231. [Google Scholar] [CrossRef]
- Saab, C.Y.; Wang, J.; Gu, C.; Garner, K.N.; Al-Chaer, E.D. Microglia: A newly discovered role in visceral hypersensitivity? Neuron. Glia Biol. 2006, 2, 271–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Yu, L.; Chen, Z.Y.; Zhu, J.S.; Hua, R.; Qin, X.; Cao, J.L.; Zhang, Y.M. Activation of corticotropin-releasing factor neurons and microglia in paraventricular nucleus precipitates visceral hypersensitivity induced by colorectal distension in rats. Brain Behav. Immun. 2016, 55, 93–104. [Google Scholar] [CrossRef]
- Yuan, T.; Orock, A.; Greenwood-Van Meerveld, B. Amygdala microglia modify neuronal plasticity via complement C1q/C3-CR3 signaling and contribute to visceral pain in a rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G1081–G1092. [Google Scholar] [CrossRef] [PubMed]
- Pollandt, S.; Liu, J.; Orozco-Cabal, L.; Grigoriadis, D.E.; Vale, W.W.; Gallagher, J.P.; Shinnick-Gallagher, P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur. J. Neurosci. 2006, 24, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Neugebauer, V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J. Neurosci. 2008, 28, 3861–3876. [Google Scholar] [CrossRef] [Green Version]
- Hipólito, L.; Fakira, A.K.; Cabañero, D.; Blandón, R.; Carlton, S.M.; Morón, J.A.; Melyan, Z. In vivo activation of the SK channel in the spinal cord reduces the NMDA receptor antagonist dose needed to produce antinociception in an inflammatory pain model. Pain 2015, 156, 849–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veinante, P.; Yalcin, I.; Barrot, M. The amygdala between sensation and affect: A role in pain. J. Mol. Psychiatr. 2013, 1, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef]
- Cavanagh, H.M.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; Melo, D.A.; Donadio, M.V.; Nunes, F.B.; de Azambuja, M.S.; Santana, J.C.; Moraes, C.M.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad. Bras. Cienc. 2015, 87 (Suppl. 2), 1397–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barocelli, E.; Calcina, F.; Chiavarini, M.; Bruni, R.; Bianchi, A.; Ballabeni, V. Antinociceptive and gastroprotective effects of inhaled and orally administered Lavandula hybrida Reverchon "Grosso" essential oil. Life Sci. 2004, 76, 213–223. [Google Scholar] [CrossRef]
- Shaw, D.; Annett, J.M.; Doherty, B.; Leslie, J.C. Anxiolytic effects of lavender oil inhalation on open-field behaviour in rats. Phytomedicine 2007, 14, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Łysakowska, M.; Ciećwierz, J.; Denys, P.; Kowalczyk, E. Antibacterial activity of thyme and lavender essential oils. Med. Chem. 2011, 7, 674–689. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Canhoto, J.; Vaz, S.; Pinto, E.; Salgueiro, L. Lavandula luisieri essential oil as a source of antifungal drugs. Food Chem. 2012, 135, 1505–1510. [Google Scholar] [CrossRef]
- Malcolm, B.J.; Tallian, K. Essential oil of lavender in anxiety disorders: Ready for prime time? Ment. Health Clin. 2018, 7, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.; Norwood, K.; Leslie, J.C. Chlordiazepoxide and lavender oil alter unconditioned anxiety-induced c-fos expression in the rat brain. Behav. Brain Res. 2011, 224, 1–7. [Google Scholar] [CrossRef]
- Price, J.L. Comparative aspects of amygdala connectivity. Ann. N. Y. Acad. Sci. 2003, 985, 50–58. [Google Scholar] [CrossRef]
- LeDoux, J. The amygdala. Curr. Biol. 2007, 17, R868–R874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Hwang, B.H.; Chang, H.M.; Gu, Z.H.; Suzuki, R. c-fos gene expression is increased in the paraventricular hypothalamic nucleus of Sprague-Dawley rats with visceral pain induced by acetic acid without detectable changes of corticotropin-releasing factor mRNA: A quantitative approach with an image analysis system. Anat. Rec. 2007, 290, 406–413. [Google Scholar] [CrossRef]
- Giamberardino, M.A. Recent and forgotten aspects of visceral pain. Eur. J. Pain 1999, 3, 77–92. [Google Scholar] [CrossRef]
- Ocuno, H. Regulation and function of immediate-early genes in the brain: Beyond neuronal activity markers. Neurosci. Res. 2011, 69, 175–186. [Google Scholar] [CrossRef]
- Burstein, R.; Potrebic, S. Retrograde labeling of neurons in the spinal cord that project directly to the amygdala or the orbital cortex in the rat. J. Comp. Neurol. 1993, 335, 469–485. [Google Scholar] [CrossRef]
- Frederickson, R.C.A.; Burgis, V.; Harrell, C.E.; Edwards, J.D. Dual actions of substance P on nociception: Possible role of endogenous opioids. Science 1978, 199, 1359–1362. [Google Scholar] [CrossRef]
- Shimada, S.; Inagaki, S.; Kubota, Y.; Ogawa, N.; Shibasaki, T.; Takagi, H. Coexistence of peptides (corticotropin releasing factor/neurotensin and substance P/somatostatin) in the bed nucleus of the stria terminalis and central amygdaloid nucleus of the rat. Neuroscience 1989, 30, 377–383. [Google Scholar] [CrossRef]
- Gray, T.S. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann. N. Y. Acad. Sci. 1993, 697, 53–60. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Prado, W.A. Role of PAG in the antinociception evoked from the medial or central amygdala in rats. Brain Res. Bull. 2001, 54, 55–63. [Google Scholar] [CrossRef]
- Faber, E.S.; Delaney, A.J.; Sah, P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat. Neurosci. 2005, 8, 635–641. [Google Scholar] [CrossRef]
- Frank, M.G.; Fonken, L.K.; Watkins, L.R.; Maier, S.F. Microglia: Neuroimmune-sensors of stress. Semin. Cell Dev. Biol. 2019, 94, 176–185. [Google Scholar] [CrossRef]
- Wang, Y.L.; Han, Q.Q.; Gong, W.Q.; Pan, D.H.; Wang, L.Z.; Hu, W.; Yang, M.; Li, B.; Yu, J.; Liu, Q. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J. Neuroinflammation 2018, 15, 21. [Google Scholar] [CrossRef]
- Yuan, T.; Manohar, K.; Latorre, R.; Orock, A.; Greenwood-Van Meerveld, B. Inhibition of microglial activation in the amygdala reverses stress-induced abdominal pain in the male rat. Cell Mol. Gastroenterol. Hepatol. 2020, 10, 527–543. [Google Scholar] [CrossRef]
- Dolga, A.M.; Letsche, T.; Gold, M.; Doti, N.; Bacher, M.; Chiamvimonvat, N.; Dodel, R.; Culmsee, C. Activation of KCNN3/SK3/K(Ca)2.3 channels attenuates enhanced calcium influx and inflammatory cytokine production in activated microglia. Glia 2012, 60, 2050–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woronuk, G.; Demissie, Z.; Rheault, M.; Mahmoud, S. Biosynthesis and therapeutic properties of Lavandula essential oil constituents. Planta Med. 2011, 77, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandula angustifolia and Lavandula latifolia essential oils from Spain: Aromatic profile and bioactivities. Planta Med. 2016, 82, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Peana, A.T.; D’Aquila, P.S.; Chessa, M.L.; Moretti, M.D.; Serra, G.; Pippia, P. (-)-Linalool produces antinociception in two experimental models of pain. Eur. J. Pharmacol. 2003, 460, 37–41. [Google Scholar] [CrossRef]
- Peana, A.T.; De Montis, M.G.; Nieddu, E.; Spano, M.T.; D’Aquila, P.S.; Pippia, P. Profile of spinal and supra-spinal antinociception of (-)-linalool. Eur. J. Pharmacol. 2004, 485, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Parviz, M.; Keshavarz, M.; Javanmardi, K. CB1 receptor activation in the basolateral amygdala produces antinociception in animal models of acute and tonic nociception. Clin. Exp. Pharmacol. Physiol. 2007, 34, 439–449. [Google Scholar] [CrossRef]
- Savelev, S.; Okello, E.; Perry, N.S.; Wilkins, R.M.; Perry, E.K. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 2003, 75, 661–668. [Google Scholar] [CrossRef]
- Yamada, K.; Mimaki, Y.; Sashida, Y. Effects of inhaling the vapor of Lavandula burnatii super-derived essential oil and linalool on plasma adrenocorticotropic hormone (ACTH), catecholamine and gonadotropin levels in experimental menopausal female rats. Biol. Pharm. Bull. 2005, 28, 378–379. [Google Scholar] [CrossRef]
- Peana, A.T.; Rubattu, P.; Piga, G.G.; Fumagalli, S.; Boatto, G.; Pippia, P.; De Montis, M.G. Involvement of adenosine A1 and A2A receptors in (-)-linalool-induced antinociception. Life Sci. 2006, 78, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.A.; Werner, M.F.; Oliveira, E.C.; Burgos, L.; Pereira, P.; Brum, L.F.; Santos, A.R. Evidence for the involvement of ionotropic glutamatergic receptors on the antinociceptive effect of (-)-linalool in mice. Neurosci. Lett. 2008, 440, 299–303. [Google Scholar] [CrossRef]
- Tayebati, S.K.; Nwankwo, I.E.; Amenta, F. Intranasal drug delivery to the central nervous system: Present status and future outlook. Curr. Pharm. Des. 2013, 19, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Buchbauer, G.; Jirovetz, L.; Jäger, W.; Plank, C.; Dietrich, H. Fragrance compounds and essential oils with sedative effects upon inhalation. J. Pharm. Sci. 1993, 82, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Akahoshi, Y.; Ito, M.; Kaneko, S. Sedative effects of inhaled essential oil components of traditional fragrance Pogostemon cablin leaves and their structure-activity relationships. J. Tradit. Complement. Med. 2015, 6, 140–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javed, F.; Bello-Correa, F.O.; Nikolaidou, A.; Rossouw, P.E.; Michelogiannakis, D. Anti-nociceptive efficacy of essential oil-based extracts for the management of orofacial pain: A systematic review of available evidence. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7323–7332. [Google Scholar] [CrossRef]
- Laird, J.M.A.; Martinez-Caro, L.; Garcia-Nicas, E.; Cervero, F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 2001, 92, 335–342. [Google Scholar] [CrossRef]
- Nakagawa, T.; Katsuya, A.; Tanimoto, S.; Yamamoto, J.; Yamauchi, Y.; Minami, M.; Satoh, M. Differential patterns of c-fos mRNA expression in the amygdaloid nuclei induced by chemical somatic and visceral noxious stimuli in rats. Neurosci. Lett. 2003, 344, 197–200. [Google Scholar] [CrossRef]
- Myers, B.; Greenwood-Van Meerveld, B. Divergent effects of amygdala glucocorticoid and mineralocorticoid receptors in the regulation of visceral and somatic pain. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G295–G303. [Google Scholar] [CrossRef]
- Murphy, A.Z.; Suckow, S.K.; Johns, M.; Traub, R.J. Sex differences in the activation of the spinoparabrachial circuit by visceral pain. Physiol. Behav. 2009, 97, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Guo, Y.; Bradesi, S.; Labus, J.S.; Maarek, J.I.; Lee, K.; Winchester, W.J.; Mayer, E.A.; Holschneider, D.P. Sex differences in functional brain activation during noxious visceral stimulation in rats. Pain 2009, 145, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Premachandran, H.; Zhao, M.; Arruda-Carvalho, M. Sex differences in development of the rodent corticolimbic system. Front. Neurosci. 2020, 14, 583477. [Google Scholar] [CrossRef]
- Cibert-Goton, V.; Kung, V.W.S.; McGuire, C.; Hockley, J.R.F.; Tranter, M.M.; Dogra, H.; Belai, A.; Blackshaw, L.A.; Sanger, G.J.; Knowles, C.H.; et al. Functional and anatomical deficits in visceral nociception with age: A mechanism of silent appendicitis in the elderly? Pain 2020, 161, 773–786. [Google Scholar] [CrossRef]
- Tran, L.; Greenwood-Van Meerveld, B. Lateralized amygdala activation: Importance in the regulation of anxiety and pain behavior. Physiol. Behav. 2012, 105, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Sadler, K.E.; McQuaid, N.A.; Cox, A.C.; Behun, M.N.; Trouten, A.M.; Kolber, B.J. Divergent functions of the left and right central amygdala in visceral nociception. Pain 2017, 158, 747–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youn, S.C.; Chen, L.Y.; Chiou, R.J.; Lai, T.J.; Liao, W.C.; Mai, F.D.; Chang, H.M. Comprehensive application of time-of-flight secondary ion mass spectrometry (TOF-SIMS) for ionic imaging and bio-energetic analysis of club drug-induced cognitive deficiency. Sci. Rep. 2015, 5, 18420. [Google Scholar] [CrossRef]
- Kuo, Y.J.; Huang, Y.K.; Chou, H.C.; Pai, M.H.; Lee, A.W.; Mai, F.D.; Chang, H.M. Reduced dental calcium expression and dental mass in chronic sleep deprived rats: Combined EDS, TOF-SIMS, and micro-CT analysis. Appl. Surf. Sci. 2015, 345, 141–144. [Google Scholar] [CrossRef]
- Chang, H.M.; Mai, F.D.; Chen, B.J.; Wu, U.I.; Huang, Y.L.; Lan, C.T.; Ling, Y.C. Sleep deprivation predisposes liver to oxidative stress and phospholipid damage—A quantitative molecular imaging study. J. Anat. 2008, 212, 295–305. [Google Scholar] [CrossRef]
- Chang, H.M.; Liu, C.H.; Hsu, W.M.; Chen, L.Y.; Wang, H.P.; Wu, T.H.; Chen, K.Y.; Ho, W.H.; Liao, W.C. Proliferative effects of melatonin on Schwann cells: Implication for nerve regeneration following peripheral nerve injury. J. Pineal. Res. 2014, 56, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arjona, M.D.M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia morphological categorization in a rat model of neuroinflammation by hierarchical cluster and principal components analysis. Front. Cell Neurosci. 2014, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.C.; Greenwood-Van Meerveld, B. The pharmacology of visceral pain. Adv. Pharmacol. 2016, 75, 273–301. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.-C.; Yao, R.-A.; Chen, L.-Y.; Renn, T.-Y.; Klimenkov, I.V.; Sudakov, N.P.; Mai, F.-D.; Chen, Y.-T.; Chang, H.-M. Olfactory Stimulation Successfully Modulates the Neurochemical, Biochemical and Behavioral Phenotypes of the Visceral Pain. Molecules 2022, 27, 7659. https://doi.org/10.3390/molecules27217659
Liao W-C, Yao R-A, Chen L-Y, Renn T-Y, Klimenkov IV, Sudakov NP, Mai F-D, Chen Y-T, Chang H-M. Olfactory Stimulation Successfully Modulates the Neurochemical, Biochemical and Behavioral Phenotypes of the Visceral Pain. Molecules. 2022; 27(21):7659. https://doi.org/10.3390/molecules27217659
Chicago/Turabian StyleLiao, Wen-Chieh, Rou-An Yao, Li-You Chen, Ting-Yi Renn, Igor V. Klimenkov, Nikolay P. Sudakov, Fu-Der Mai, Yea-Tzy Chen, and Hung-Ming Chang. 2022. "Olfactory Stimulation Successfully Modulates the Neurochemical, Biochemical and Behavioral Phenotypes of the Visceral Pain" Molecules 27, no. 21: 7659. https://doi.org/10.3390/molecules27217659