Chemistry, Biosynthesis and Pharmacology of Viniferin: Potential Resveratrol-Derived Molecules for New Drug Discovery, Development and Therapy
Abstract
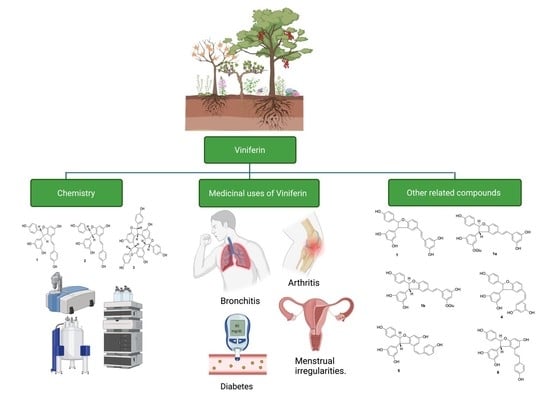
:1. Introduction
2. Chemistry
2.1. Sources and Distribution of Viniferin
2.2. Structural Characterization of Viniferin
trans-ε-Viniferin
2.3. Biosynthesis
2.4. Bioavailability and Pharmacokinetics of Viniferin
2.5. Medicinal Uses of Plants Containing Viniferin
3. Biological Properties of Viniferin
3.1. Anti-Inflammatory Effects
3.2. Antidiabetic Effects
3.3. Anticancer Effects
3.4. Anti-Angiogenic Effects
3.5. Anti-Melanogenic Effects
3.6. Anti-Obesity Effects
3.7. Antidiarrheal Effects
3.8. Neuroprotective Effects
3.9. Antioxidant Effects
3.10. Antiplasmodic Effects
3.11. Antimicrobial Effects
3.12. Antihelmintic Effects
4. Industrial Application of Viniferin
5. Structurally-Related Viniferin Molecules for New Drug Discovery and Development










6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramawat, K.G.; Mérillon, J.-M. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Riviere, C.; Pawlus, A.D.; Merillon, J.-M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Houillé, B.; Besseau, S.; Delanoue, G.; Oudin, A.; Papon, N.; Clastre, M.; Simkin, A.J.; Guerin, L.; Courdavault, V.; Giglioli-Guivarc’h, N. Composition and tissue-specific distribution of stilbenoids in grape canes are affected by downy mildew pressure in the vineyard. J. Agric. Food Chem. 2015, 63, 8472–8477. [Google Scholar] [CrossRef] [PubMed]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Barrio, R.; Beltrán, D.; Cantos, E.; Gil, M.I.; Espín, J.C.; Tomás-Barberán, F.A. Comparison of ozone and UV-C treatments on the postharvest stilbenoid monomer, dimer, and trimer induction in var. ‘Superior’ white table grapes. J. Agric. Food Chem. 2006, 54, 4222–4228. [Google Scholar] [CrossRef]
- Dilshara, M.G.; Lee, K.-T.; Kim, H.J.; Lee, H.-J.; Choi, Y.H.; Lee, C.-M.; Kim, L.K.; Kim, G.-Y. Anti-inflammatory mechanism of α-viniferin regulates lipopolysaccharide-induced release of proinflammatory mediators in BV2 microglial cells. Cell. Immunol. 2014, 290, 21–29. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, L.; Huang, X.; Jia, Q.; Wang, W.; Gao, M.; Jia, X.; Chang, Y.; Ouyang, H.; He, J. Pharmacokinetic and bioavailability studies of α-viniferin after intravenous and oral administration to rats. J. Pharm. Biomed. Anal. 2020, 188, 113376. [Google Scholar] [CrossRef]
- Wilkens, A.; Paulsen, J.; Wray, V.; Winterhalter, P. Structures of two novel trimeric stilbenes obtained by horseradish peroxidase catalyzed biotransformation of trans-resveratrol and (−)-ε-viniferin. J. Agric. Food Chem. 2010, 58, 6754–6761. [Google Scholar] [CrossRef]
- Mattio, L.M.; Marengo, M.; Parravicini, C.; Eberini, I.; Dallavalle, S.; Bonomi, F.; Iametti, S.; Pinto, A. Inhibition of Pancreatic α-amylase by Resveratrol derivatives: Biological activity and molecular modelling evidence for cooperativity between viniferin enantiomers. Molecules 2019, 24, 3225. [Google Scholar] [CrossRef] [Green Version]
- Pezet, R.; Perret, C.; Jean-Denis, J.B.; Tabacchi, R.; Gindro, K.; Viret, O. δ-Viniferin, a resveratrol dehydrodimer: One of the major stilbenes synthesized by stressed grapevine leaves. J. Agric. Food Chem. 2003, 51, 5488–5492. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, J.-H.; Ryu, S.Y.; Joo, S.W.; Cho, M.H.; Lee, J. Inhibition of Pseudomonas aeruginosa and Escherichia coli O157: H7 biofilm formation by plant metabolite ε-viniferin. J. Agric. Food Chem. 2013, 61, 7120–7126. [Google Scholar] [CrossRef]
- Courtois, A.; Garcia, M.; Krisa, S.; Atgié, C.; Sauvant, P.; Richard, T.; Faure, C. Encapsulation of ε-viniferin in onion-type multi-lamellar liposomes increases its solubility and its photo-stability and decreases its cytotoxicity on Caco-2 intestinal cells. Food Funct. 2019, 10, 2573–2582. [Google Scholar] [CrossRef]
- Saad, N.M.; Sekar, M.; Gan, S.H.; Lum, P.T.; Vaijanathappa, J.; Ravi, S. Resveratrol: Latest scientific evidences of its chemical, biological activities and therapeutic potentials. Pharmacog. J. 2020, 12, 1779–1791. [Google Scholar] [CrossRef]
- Empl, M.T.; Albers, M.; Wang, S.; Steinberg, P. The Resveratrol Tetramer r-Viniferin Induces a Cell Cycle Arrest Followed by Apoptosis in the Prostate Cancer Cell Line LNCaP. Phytother. Res. 2015, 29, 1640–1645. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, D.; Li, S.; Xuan, X.; Zhang, L.; Li, Y.; Guo, F. Inhibitors of BRD4 protein from the roots of Astilbe grandis stapf ex EH Wilson. Nat. Prod. Res. 2021, 35, 2044–2050. [Google Scholar] [CrossRef]
- Lam, S.-H.; Chen, J.-M.; Tsai, S.-F.; Lee, S.-S. Chemical investigation on the root bark of Bombax malabarica. Fitoterapia 2019, 139, 104376. [Google Scholar] [CrossRef]
- Sim, J.; Jang, H.W.; Song, M.; Kim, J.H.; Lee, S.H.; Lee, S. Potent inhibitory effect of alpha-viniferin on human cytochrome P450. Food Chem. Toxicol. 2014, 69, 276–280. [Google Scholar] [CrossRef]
- Jeong, W.; Ahn, E.-K.; Oh, J.S.; Hong, S.S. Caragasinin C: A new oligostilbene from the roots of Caragana sinica. J. Asian Nat. Prod. Res. 2017, 19, 1143–1147. [Google Scholar] [CrossRef]
- Jin, Q.; Han, X.H.; Hong, S.S.; Lee, C.; Choe, S.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Antioxidative oligostilbenes from Caragana sinica. Bioorganic Med. Chem. Lett. 2012, 22, 973–976. [Google Scholar] [CrossRef]
- Cho, Y.R.; Ahn, E.K.; Park, Y.J.; Park, K.; Hong, S.S.; Seo, D.W.; Oh, J.S. A novel role for α-viniferin in suppressing angiogenesis by blocking the VEGFR-2/p70S6K signaling pathway. Phytother. Res. 2020, 34, 2697–2705. [Google Scholar] [CrossRef]
- Schuck, F.; Schmitt, U.; Reinhardt, S.; Freese, C.; Lee, I.-S.; Thines, E.; Efferth, T.; Endres, K. Extract of Caragana sinica as a potential therapeutic option for increasing alpha-secretase gene expression. Phytomedicine 2015, 22, 1027–1036. [Google Scholar] [CrossRef]
- Roy, B.; Giri, B.R. α-Viniferin-induced structural and functional alterations in Raillietina echinobothrida, a poultry tapeworm. Microsc. Microanal. 2015, 21, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Shin, N.-H.; Kang, S.-H.; Park, J.S.; Chung, S.R.; Min, K.R.; Kim, Y. α-Viniferin: A prostaglandin H2 synthase inhibitor from root of Carex humilis. Planta Med. 1998, 64, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.Y.; Kim, B.H.; Lee, M.K.; Yun, Y.-P.; Lee, S.H.; Min, K.R.; Kim, Y. Anti-inflammatory effect of the oligomeric stilbene α-viniferin and its mode of the action through inhibition of cyclooxygenase-2 and inducible nitric oxide synthase. Planta Med. 2003, 69, 710–714. [Google Scholar] [PubMed]
- Arora, J.; Roat, C.; Goyal, S.; Ramawat, K.G. High stilbenes accumulation in root cultures of Cayratia trifolia (L.) Domin grown in shake flasks. Acta Physiol. Plant. 2009, 31, 1307–1312. [Google Scholar] [CrossRef]
- Bala, A.; Kollmann, A.; Ducrot, P.H.; Majira, A.; Kerhoas, L.; Leroux, P.; Delorme, R.; Einhorn, J. Cis ε-viniferin: A New Antifungal Resveratrol Dehydrodimer from Cyphostemma crotalarioides Roots. J. Phytopathol. 2000, 148, 29–32. [Google Scholar] [CrossRef]
- Lulan, T.Y.; Fatmawati, S.; Santoso, M.; Ersam, T. α-Viniferin as a potential antidiabetic and antiplasmodial extracted from Dipterocarpus littoralis. Heliyon 2020, 6, e04102. [Google Scholar] [CrossRef]
- Ahmat, N.; Wibowo, A.; Mohamad, S.A.S.; Low, A.L.M.; Sufian, A.S.; Yusof, M.I.M.; Latip, J. A new symmetrical tetramer oligostilbenoid containing tetrahydrofuran ring from the stem bark of Dryobalanops lanceolata. J. Asian Nat. Prod. Res. 2014, 16, 1099–1107. [Google Scholar] [CrossRef]
- Moriyama, H.; Moriyama, M.; Ninomiya, K.; Morikawa, T.; Hayakawa, T. Inhibitory effects of oligostilbenoids from the bark of Shorea roxburghii on malignant melanoma cell growth: Implications for novel topical anticancer candidates. Biol. Pharm. Bull. 2016, 39, 1675–1682. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.M.; Huang, B.; Tan, S.H.; Shi, D.H.; Song, Y.C.; Tan, R.X. Bioactive oligostilbenoids from the stem bark of Hopea exalata. J. Nat. Prod. 2006, 69, 1800–1802. [Google Scholar] [CrossRef]
- Prabha, B.; Sini, S.; Priyadarshini, T.; Sasikumar, P.; Gopalan, G.; Joseph, J.P.; Jithin, M.; Sivan, V.; Jayamurthy, P.; Radhakrishnan, K. Anti-inflammatory effect and mechanism of action of ellagic acid-3, 3′, 4-trimethoxy-4′-O-α-L-rhamnopyranoside isolated from Hopea parviflora in lipopolysaccharide-stimulated RAW 264.7 macrophages. Nat. Prod. Res. 2021, 35, 3156–3160. [Google Scholar] [CrossRef]
- Sasikumar, P.; Lekshmy, K.; Sini, S.; Prabha, B.; Kumar, N.A.; Sivan, V.; Jithin, M.; Jayamurthy, P.; Shibi, I.; Radhakrishnan, K. Isolation and characterization of resveratrol oligomers from the stem bark of Hopea ponga (Dennst.) Mabb. and their antidiabetic effect by modulation of digestive enzymes, protein glycation and glucose uptake in L6 myocytes. J. Ethnopharmacol. 2019, 236, 196–204. [Google Scholar] [CrossRef]
- Keckeis, K. Resveratrol-type oligostilbenes from Iris clarkei antagonize 20-hydroxyecdysone action in the Drosophila melanogaster BII cell line. Cell. Mol. Life Sci. CMLS 2000, 57, 333–336. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, S.S.; Kang, K.B.; Ryu, B.; Park, E.; Huh, J.; Jeon, W.K.; Chae, H.-S.; Oh, W.K.; Kim, J. Combined MS/MS-NMR annotation guided discovery of Iris lactea var. chinensis seed as a source of viral neuraminidase inhibitory polyphenols. Molecules 2020, 25, 3383. [Google Scholar] [CrossRef]
- Ha, M.T.; Park, D.H.; Shrestha, S.; Kim, M.; Kim, J.A.; Woo, M.H.; Choi, J.S.; Min, B.S. PTP1B inhibitory activity and molecular docking analysis of stilbene derivatives from the rhizomes of Rheum undulatum L. Fitoterapia 2018, 131, 119–126. [Google Scholar] [CrossRef]
- Yuk, H.J.; Ryu, H.W.; Jeong, S.H.; Curtis-Long, M.J.; Kim, H.J.; Wang, Y.; Song, Y.H.; Park, K.H. Profiling of neuraminidase inhibitory polyphenols from the seeds of Paeonia lactiflora. Food Chem. Toxicol. 2013, 55, 144–149. [Google Scholar] [CrossRef]
- Tian, X.; Guo, S.; Zhang, S.; Li, P.; Wang, T.; Ho, C.T.; Pan, M.H.; Bai, N. Chemical characterization of main bioactive constituents in Paeonia ostii seed meal and GC-MS analysis of seed oil. J. Food Biochem. 2020, 44, e13088. [Google Scholar] [CrossRef]
- He, Y.-K.; Cen, X.-t.; Liu, S.-s.; Lu, H.-d.; He, C.-n. Protective effects of ten oligostilbenes from Paeonia suffruticosa seeds on interleukin-1β-induced rabbit osteoarthritis chondrocytes. BMC Chem. 2019, 13, 72. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-B.; Wang, A.-G.; Ji, T.-F.; Su, Y.-L. Two new oligostilbenes from the stem of Parthenocissus quinquefolia. J. Asian Nat. Prod. Res. 2014, 16, 275–280. [Google Scholar] [CrossRef]
- Liu, W.-B.; Hu, L.; Hu, Q.; Chen, N.-N.; Yang, Q.-S.; Wang, F.-F. New resveratrol oligomer derivatives from the roots of Rheum lhasaense. Molecules 2013, 18, 7093–7102. [Google Scholar] [CrossRef] [Green Version]
- Zawawi, N.; Ahmat, N.; Mazatulikhma, M.; Shafiq, R.; Wahid, N.; Sufian, A. Bioactive oligostilbenoids from Shorea maxwelliana King and their chemotaxonomic significance. Nat. Prod. Res. 2013, 27, 1589–1593. [Google Scholar] [CrossRef]
- Hadi, S.; Noviany, N. The isolation of hopeaphenol, a tetramer stilbene, from Shorea ovalis Blume. Adv. Nat. Appl. Sci. 2009, 3, 107–112. [Google Scholar]
- Aminah, N.S.; Achmad, S.A.; Aimi, N.; Ghisalberti, E.L.; Hakim, E.H.; Kitajima, M.; Syah, Y.M.; Takayama, H. Diptoindonesin A, a new C-glucoside of ε-viniferin from Shorea seminis (Dipterocarpaceae). Fitoterapia 2002, 73, 501–507. [Google Scholar] [CrossRef]
- Azmin, N.F.N.; Ahmat, N.; Syah, Y.M.; Zawawi, N.K.N.A.; Yusof, M.I.M. A new stilbenoid compound from the lianas of Gnetum microcarpum. Nat. Prod. Commun. 2014, 9, 1934578X1400901221. [Google Scholar] [CrossRef] [Green Version]
- Kiselev, K.V.; Aleynova, O.A.; Grigorchuk, V.P.; Dubrovina, A.S. Stilbene accumulation and expression of stilbene biosynthesis pathway genes in wild grapevine Vitis amurensis Rupr. Planta 2017, 245, 151–159. [Google Scholar] [CrossRef]
- Morikawa, T.; Chaipech, S.; Matsuda, H.; Hamao, M.; Umeda, Y.; Sato, H.; Tamura, H.; Kon’i, H.; Ninomiya, K.; Yoshikawa, M. Antidiabetogenic oligostilbenoids and 3-ethyl-4-phenyl-3, 4-dihydroisocoumarins from the bark of Shorea roxburghii. Bioorganic Med. Chem. 2012, 20, 832–840. [Google Scholar] [CrossRef]
- Ha, D.T.; Long, P.T.; Hien, T.T.; Tuan, D.T.; An, N.T.T.; Khoi, N.M.; Van Oanh, H.; Hung, T.M. Anti-inflammatory effect of oligostilbenoids from Vitis heyneana in LPS-stimulated RAW 264.7 macrophages via suppressing the NF-κB activation. Chem. Cent. J. 2018, 12, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, C.; Lemaire, J.; Auger, H.; Guilleret, A.; Reynaud, R.; Clément, C.; Courot, E.; Taidi, B. Optimize, modulate, and scale-up resveratrol and resveratrol dimers bioproduction in Vitis labrusca L. cell suspension from flasks to 20 L bioreactor. Plants 2019, 8, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roat, C.; Saraf, M. Isolation and characterization of t-resveratrol and α-viniferin, a bioactive secondary metabolite of an endophytic fungus Aspergillus stellifer ab4, from Vitis vinifera. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 708–713. [Google Scholar] [CrossRef]
- Nopo-Olazabal, C.; Hubstenberger, J.; Nopo-Olazabal, L.; Medina-Bolivar, F. Antioxidant activity of selected stilbenoids and their bioproduction in hairy root cultures of muscadine grape (Vitis rotundifolia Michx.). J. Agric. Food Chem. 2013, 61, 11744–11758. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Lin, Y.-K.; Yang, S.-C.; Alalaiwe, A.; Lin, C.-J.; Fang, J.-Y.; Lin, C.-F. Percutaneous absorption of resveratrol and its oligomers to relieve psoriasiform lesions: In silico, in vitro and in vivo evaluations. Int. J. Pharm. 2020, 585, 119507. [Google Scholar] [CrossRef]
- Lu, Y.-L.; Lin, S.-Y.; Fang, S.-U.; Hsieh, Y.-Y.; Chen, C.-R.; Wen, C.-L.; Chang, C.-I.; Hou, W.-C. Hot-water extracts from roots of Vitis thunbergii var. taiwaniana and identified ε-viniferin improve obesity in high-fat diet-induced mice. J. Agric. Food Chem. 2017, 65, 2521–2529. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Wang, K.-T.; Chen, L.-G.; Lee, C.-J.; Tseng, S.-H.; Wang, C.-C. Anti-inflammatory effects of Vitis thunbergii var. taiwaniana on knee damage associated with arthritis. J. Med. Food 2014, 17, 479–486. [Google Scholar]
- Sáez, V.; Pastene, E.; Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Gómez, M.V.; Theoduloz, C.; Riquelme, S.; von Baer, D. Oligostilbenoids in Vitis vinifera L. Pinot Noir grape cane extract: Isolation, characterization, in vitro antioxidant capacity and anti-proliferative effect on cancer cells. Food Chem. 2018, 265, 101–110. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Becker, L.; Bellow, S.; Carré, V.; Latouche, G.; Poutaraud, A.; Merdinoglu, D.; Brown, S.C.; Cerovic, Z.G.; Chaimbault, P. Correlative analysis of fluorescent phytoalexins by mass spectrometry imaging and fluorescence microscopy in grapevine leaves. Anal. Chem. 2017, 89, 7099–7106. [Google Scholar] [CrossRef]
- Bruisson, S.; Maillot, P.; Schellenbaum, P.; Walter, B.; Gindro, K.; Deglène-Benbrahim, L. Arbuscular mycorrhizal symbiosis stimulates key genes of the phenylpropanoid biosynthesis and stilbenoid production in grapevine leaves in response to downy mildew and grey mould infection. Phytochemistry 2016, 131, 92–99. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E.; Puertas, B.; Richard, T. Daily preharvest UV-C light maintains the high stilbenoid concentration in grapes. J. Agric. Food Chem. 2016, 64, 5139–5147. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Ewald, P.; Yasui, Y.; Yokokawa, H.; Wagner, A.E.; Matsugo, S.; Winterhalter, P.; Rimbach, G. Chemical characterization, free radical scavenging, and cellular antioxidant and anti-inflammatory properties of a stilbenoid-rich root extract of Vitis vinifera. Oxidative Med. Cell. Longev. 2016, 2016, 8591286. [Google Scholar] [CrossRef] [Green Version]
- Qsaib, S.; Mateus, N.; Ikbal, F.E.-z.; Rifai, L.A.; De Freitas, V.; Koussa, T. Direct identification and characterization of phenolic compounds from crude extracts of buds and internodes of grapevine (Vitis vinifera cv Merlot). Nat. Prod. Commun. 2014, 9, 1934578X1400901110. [Google Scholar] [CrossRef] [Green Version]
- Lambert, C.; Richard, T.; Renouf, E.; Bisson, J.; Waffo-Téguo, P.; Bordenave, L.; Ollat, N.; Mérillon, J.-M.; Cluzet, S. Comparative analyses of stilbenoids in canes of major Vitis vinifera L. cultivars. J. Agric. Food Chem. 2013, 61, 11392–11399. [Google Scholar] [CrossRef]
- Pawlus, A.D.; Waffo-Téguo, P.; Shaver, J.; Mérillon, J.-M. Stilbenoid chemistry from wine and the genus Vitis, a review. Oeno One 2012, 46, 57–111. [Google Scholar] [CrossRef] [Green Version]
- Privat, C.; Telo, J.P.; Bernardes-Genisson, V.; Vieira, A.; Souchard, J.P.; Nepveu, F. Antioxidant properties of trans-ε-viniferin as compared to stilbene derivatives in aqueous and nonaqueous media. J. Agric. Food Chem. 2002, 50, 1213–1217. [Google Scholar] [CrossRef]
- Sahidin, I.; Wahyuni, W.; Malaka, M.; Imran, I. Antibacterial and cytotoxic potencies of stilbene oligomers from stem barks of baoti (Dryobalanops lanceolata) growing in Kendari, Indonesia. Asian J. Pharm. Clin. Res 2017, 10, 139–143. [Google Scholar]
- Kitanaka, S.; Ikezawa, T.; Yasukawa, K.; Yamanouchi, S.; Takida, M.; Sung, H.K.; Kim, I.H. (+)-α-viniferin, an anti-inflammatory compound from Caragana chamlagu root. Chem. Pharm. Bull. 1990, 38, 432–435. [Google Scholar] [CrossRef] [Green Version]
- Teng, B.-H.; Zhu, Q.-B.; Fan, Y.-Y.; Yao, C.-S. Total synthesis of the active resveratrol dimer dehydro-δ-viniferin. J. Asian Nat. Prod. Res. 2020, 22, 947–955. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R. A new class of phytoalexins from grapevines. Experientia 1977, 33, 151–152. [Google Scholar] [CrossRef]
- Courtois, A.; Jourdes, M.; Dupin, A.; Lapèze, C.; Renouf, E.; Biais, B.; Teissedre, P.-L.; Mérillon, J.-M.; Richard, T.; Krisa, S. In vitro glucuronidation and sulfation of ε-viniferin, a resveratrol dimer, in humans and rats. Molecules 2017, 22, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willenberg, I.; Brauer, W.; Empl, M.T.; Schebb, N.H. Development of a rapid LC-UV method for the investigation of chemical and metabolic stability of resveratrol oligomers. J. Agric. Food Chem. 2012, 60, 7844–7850. [Google Scholar] [CrossRef] [PubMed]
- Courtois, A.; Atgié, C.; Marchal, A.; Hornedo-Ortega, R.; Lapèze, C.; Faure, C.; Richard, T.; Krisa, S. Tissular distribution and metabolism of trans-ε-viniferin after intraperitoneal injection in rat. Nutrients 2018, 10, 1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, P.; Lei, Y.; Zhang, T.; Ma, C.; Jin, B.; Li, T. Pharmacokinetics, bioavailability, metabolism and excretion of δ-viniferin in rats. Acta Pharm. Sin. B 2016, 6, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, H.; Asao, Y.; Nakamura, S.; Hamao, M.; Sugimoto, S.; Hongo, M.; Pongpiriyadacha, Y.; Yoshikawa, M. Antidiabetogenic constituents from the Thai traditional medicine Cotylelobium melanoxylon. Chem. Pharm. Bull. 2009, 57, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Ninomiya, K.; Chaipech, S.; Kunikata, Y.; Yagi, R.; Pongpiriyadacha, Y.; Muraoka, O.; Morikawa, T. Quantitative determination of stilbenoids and dihydroisocoumarins in Shorea roxburghii and evaluation of their hepatoprotective activity. Int. J. Mol. Sci. 2017, 18, 451. [Google Scholar] [CrossRef] [Green Version]
- Ha, D.T.; Chen, Q.C.; Hung, T.M.; Youn, U.J.; Ngoc, T.M.; Thuong, P.T.; Kim, H.J.; Seong, Y.H.; Min, B.S.; Bae, K. Stilbenes and oligostilbenes from leaf and stem of Vitis amurensis and their cytotoxic activity. Arch. Pharmacal Res. 2009, 32, 177–183. [Google Scholar] [CrossRef]
- Sung, S.H.; Kang, S.Y.; Lee, K.Y.; Park, M.J.; Kim, J.H.; Park, J.H.; Kim, Y.C.; Kim, J.; Kim, Y.C. (+)-Alpha-viniferin, a stilbene trimer from Caragana chamlague, inhibits acetylcholinesterase. Biol. Pharm. Bull. 2002, 25, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Vion, E.; Page, G.; Bourdeaud, E.; Paccalin, M.; Guillard, J.; Bilan, A.R. Trans ε-viniferin is an amyloid-β disaggregating and anti-inflammatory drug in a mouse primary cellular model of Alzheimer’s disease. Mol. Cell. Neurosci. 2018, 88, 1–6. [Google Scholar] [CrossRef]
- Chung, E.Y.; Roh, E.; Kwak, J.-A.; Lee, H.-S.; Lee, S.H.; Lee, C.-K.; Han, S.-B.; Kim, Y. α-Viniferin suppresses the signal transducer and activation of transcription-1 (STAT-1)–inducible inflammatory genes in Interferon-γ–stimulated macrophages. J. Pharmacol. Sci. 2010, 112, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Zhang, Y.; Yao, X.; Wu, Q.; Wei, M.; Yan, Z. ε-Viniferin, a promising natural oligostilbene, ameliorates hyperglycemia and hyperlipidemia by activating AMPK in vivo. Food Funct. 2020, 11, 10084–10093. [Google Scholar] [CrossRef]
- Oranje, P.; Gouka, R.; Burggraaff, L.; Vermeer, M.; Chalet, C.; Duchateau, G.; van der Pijl, P.; Geldof, M.; de Roo, N.; Clauwaert, F. Novel natural and synthetic inhibitors of solute carriers SGLT1 and SGLT2. Pharmacol. Res. Perspect. 2019, 7, e00504. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, F.; Apaydın, E.; Önder, N.İ.; Şen, M.; Ayrım, A.; Öğünç, Y.; İncesu, Z. Apoptotic effects of ε-viniferin in combination with cis-platin in C6 cells. Cytotechnology 2018, 70, 1061–1073. [Google Scholar] [CrossRef]
- Özdemir, F.; Akalın, G.; Şen, M.; Önder, N.I.; Işcan, A.; Kutlu, H.M.; Incesu, Z. Towards Novel anti-tumor strategies for hepatic cancer: ɛ-Viniferin in combination with vincristine displays pharmacodynamic synergy at lower doses in HepG2 Cells. OMICS J. Integr. Biol. 2014, 18, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Aja, I.; Ruiz-Larrea, M.B.; Courtois, A.; Krisa, S.; Richard, T.; Ruiz-Sanz, J.-I. Screening of natural stilbene oligomers from Vitis vinifera for anticancer activity on human hepatocellular carcinoma cells. Antioxidants 2020, 9, 469. [Google Scholar] [CrossRef]
- Empl, M.; Macke, S.; Winterhalter, P.; Puff, C.; Lapp, S.; Stoica, G.; Baumgärtner, W.; Steinberg, P. The growth of the canine glioblastoma cell line D-GBM and the canine histiocytic sarcoma cell line DH82 is inhibited by the resveratrol oligomers hopeaphenol and r2-viniferin. Vet. Comp. Oncol. 2014, 12, 149–159. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Gromek, S.; Niesen, D.; Seeram, N.P.; Henry, G.E. Resveratrol oligomers isolated from Carex species inhibit growth of human colon tumorigenic cells mediated by cell cycle arrest. J. Agric. Food Chem. 2011, 59, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.-y.; Gong, F.-K.; Chen, Z.-H.; Li, Z.-X.; Zhang, B. System Prediction and Validation of TCM for Chronic Myeloid Leukemia Treatment from the Perspective of Low-Toxicity Chemotherapy: A Stilbene α-Viniferin Has a Proapoptotic Effect on K562 Cells via the Mitochondrial Pathway. Evid. Based Complementary Altern. Med. 2020, 2020, 1986962. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Liu, X.; Chen, L.; Lv, J.-M.; Qu, F.-J.; Pan, X.-W.; Li, L.; Cui, X.-G.; Gao, Y.; Xu, D.-F. α-Viniferin activates autophagic apoptosis and cell death by reducing glucocorticoid receptor expression in castration-resistant prostate cancer cells. Med. Oncol. 2018, 35, 105. [Google Scholar] [CrossRef] [PubMed]
- Nivelle, L.; Aires, V.; Rioult, D.; Martiny, L.; Tarpin, M.; Delmas, D. Molecular analysis of differential antiproliferative activity of resveratrol, epsilon viniferin and labruscol on melanoma cells and normal dermal cells. Food Chem. Toxicol. 2018, 116, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Nakamoto, Y.; Hisatome, T.; Yoshida, S.; Miyazaki, H. Resveratrol and its dimers ε-viniferin and δ-viniferin in red wine protect vascular endothelial cells by a similar mechanism with different potency and efficacy. Kaohsiung J. Med. Sci. 2020, 36, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Arraki, K.; Totoson, P.; Decendit, A.; Badoc, A.; Zedet, A.; Jolibois, J.; Pudlo, M.; Demougeot, C.; Girard-Thernier, C. Cyperaceae species are potential sources of natural mammalian arginase inhibitors with positive effects on vascular function. J. Nat. Prod. 2017, 80, 2432–2438. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Lu, Y.-L.; Wang, G.-J.; Chen, L.-G.; Wen, C.-L.; Hou, W.-C. Ethanolic extracts and isolated compounds from small-leaf grape (Vitis thunbergii var. taiwaniana) with antihypertensive activities. J. Agric. Food Chem. 2012, 60, 7435–7441. [Google Scholar] [CrossRef]
- Zghonda, N.; Yoshida, S.; Ezaki, S.; Otake, Y.; Murakami, C.; Mliki, A.; Ghorbel, A.; Miyazaki, H. ε-Viniferin is more effective than its monomer resveratrol in improving the functions of vascular endothelial cells and the heart. Biosci. Biotechnol. Biochem. 2012, 76, 954–960. [Google Scholar] [CrossRef] [Green Version]
- Yun, C.-Y.; Ko, S.M.; Choi, Y.P.; Kim, B.J.; Lee, J.; Kim, J.M.; Kim, J.Y.; Song, J.Y.; Kim, S.-H.; Hwang, B.Y. α-Viniferin improves facial hyperpigmentation via accelerating feedback termination of cAMP/PKA-signaled phosphorylation circuit in facultative melanogenesis. Theranostics 2018, 8, 2031. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Jiang, Y.; Zhang, B.; Yang, H.; Ma, T. Resveratrol dimer trans-ε-viniferin prevents rotaviral diarrhea in mice by inhibition of the intestinal calcium-activated chloride channel. Pharmacol. Res. 2018, 129, 453–461. [Google Scholar] [CrossRef]
- Yu, B.; Xie, R.; Jin, L.; Tian, X.; Niu, Y.; Ma, T.; Yang, H. trans-δ-Viniferin inhibits Ca2+-activated Cl− channels and improves diarrhea symptoms. Fitoterapia 2019, 139, 104367. [Google Scholar] [CrossRef]
- Caillaud, M.; Guillard, J.; Richard, D.; Milin, S.; Chassaing, D.; Paccalin, M.; Page, G.; Rioux Bilan, A. Trans ε viniferin decreases amyloid deposits and inflammation in a mouse transgenic Alzheimer model. PLoS ONE 2019, 14, e0212663. [Google Scholar] [CrossRef]
- Richard, T.; Poupard, P.; Nassra, M.; Papastamoulis, Y.; Iglésias, M.-L.; Krisa, S.; Waffo-Teguo, P.; Mérillon, J.-M.; Monti, J.-P. Protective effect of ε-viniferin on β-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorganic Med. Chem. 2011, 19, 3152–3155. [Google Scholar] [CrossRef]
- Fu, J.; Jin, J.; Cichewicz, R.H.; Hageman, S.A.; Ellis, T.K.; Xiang, L.; Peng, Q.; Jiang, M.; Arbez, N.; Hotaling, K. trans-(−)-ε-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington disease. J. Biol. Chem. 2012, 287, 24460–24472. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Ma, Y.; Feng, J. Neuroprotective mechanisms of ε-viniferin in a rotenone-induced cell model of Parkinson’s disease: Significance of SIRT3-mediated FOXO3 deacetylation. Neural Regen. Res. 2020, 15, 2143. [Google Scholar]
- Li, X.; Xie, Y.; Xie, H.; Yang, J.; Chen, D. π-π Conjugation Enhances Oligostilbene’s Antioxidant Capacity: Evidence from α-Viniferin and Caraphenol A. Molecules 2018, 23, 694. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, E.J.; Bae, S.J.; Shim, S.M.; Park, H.D.; Rhee, C.H.; Park, J.H.; Choi, S.W. Cytotoxic and antimutagenic stilbenes from seeds of Paeonia lactiflora. Arch. Pharmacal Res. 2002, 25, 293–299. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Multifaceted approach to resveratrol bioactivity: Focus on antioxidant action, cell signaling and safety. Oxidative Med. Cell. Longev. 2010, 3, 86–100. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, E.J.; Cho, S.H.; Chung, S.K.; Park, H.D.; Choi, S.W. Antioxidative activity of resveratrol and its derivatives isolated from seeds of Paeonia lactiflora. Biosci. Biotechnol. Biochem. 2002, 66, 1990–1993. [Google Scholar] [CrossRef] [Green Version]
- Zekar, L.; Sharman, T. Plasmodium Falciparum Malaria; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- WHO. Antimicrobial Resistance. Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 25 May 2022).
- Schnee, S.; Queiroz, E.F.; Voinesco, F.; Marcourt, L.; Dubuis, P.-H.; Wolfender, J.-L.; Gindro, K. Vitis vinifera canes, a new source of antifungal compounds against Plasmopara viticola, Erysiphe necator, and Botrytis cinerea. J. Agric. Food Chem. 2013, 61, 5459–5467. [Google Scholar] [CrossRef]
- Yadav, M.K.; Mailar, K.; Nagarajappa Masagalli, J.; Chae, S.-W.; Song, J.-J.; Choi, W.J. Ruthenium Chloride—Induced Oxidative Cyclization of Trans-Resveratrol to (±)-ε-Viniferin and Antimicrobial and Antibiofilm Activity Against Streptococcus pneumoniae. Front. Pharmacol. 2019, 10, 890. [Google Scholar] [CrossRef] [Green Version]
- Mattio, L.M.; Pinna, C.; Catinella, G.; Musso, L.; Pedersen, K.J.; Krogfelt, K.A.; Dallavalle, S.; Pinto, A. Synthesis and Antimicrobial Activity of δ-Viniferin Analogues and Isosteres. Molecules 2021, 26, 7594. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Asrani, R.K.; Patial, V. Listeria monocytogenes: A food-borne pathogen. In Foodborne Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 157–192. [Google Scholar]
- Rahim, M.A.; Seo, H.; Kim, S.; Jeong, Y.K.; Tajdozian, H.; Kim, M.; Lee, S.; Song, H.-Y. A Clinical Trial to Evaluate the Efficacy of α-Viniferin in Staphylococcus aureus–Specific Decolonization without Depleting the Normal Microbiota of Nares. Pol. J. Microbiol. 2021, 70, 117. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Vrhovsek, U.; Malacarne, G.; Masuero, D.; Zulini, L.; Stefanini, M.; Moser, C.; Velasco, R.; Guella, G. Profiling of resveratrol oligomers, important stress metabolites, accumulating in the leaves of hybrid Vitis vinifera (Merzling× Teroldego) genotypes infected with Plasmopara viticola. J. Agric. Food Chem. 2011, 59, 5364–5375. [Google Scholar] [CrossRef] [PubMed]
- Gabaston, J.; Cantos-Villar, E.; Biais, B.; Waffo-Teguo, P.; Renouf, E.; Corio-Costet, M.-F.; Richard, T.; Mérillon, J.-M. Stilbenes from Vitis vinifera L. waste: A sustainable tool for controlling Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Roy, B. Resveratrol-and α-viniferin-induced alterations of acetylcholinesterase and nitric oxide synthase in Raillietina echinobothrida. Parasitol. Res. 2015, 114, 3775–3781. [Google Scholar] [CrossRef]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol derivatives as potential treatments for Alzheimer’s and Parkinson’s disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef]
- Noviello, M.; Caputi, A.F.; Squeo, G.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Vine Shoots as a Source of Trans-Resveratrol and ε-Viniferin: A Study of 23 Italian Varieties. Foods 2022, 11, 553. [Google Scholar] [CrossRef]
- Jeandet, P.; Chaudruc, D.; Robillard, B.; Peters, F.; Tusseau, D.; Conreux, A.; Duteurtre, B. Determination of the trans-resveratrol content of Champagne wines by reversed-phase HPLC. OENO One 2006, 40, 117–119. [Google Scholar] [CrossRef]
- Malinowska, M.A.; Billet, K.; Drouet, S.; Munsch, T.; Unlubayir, M.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Lanoue, A. Grape cane extracts as multifunctional rejuvenating cosmetic ingredient: Evaluation of sirtuin activity, tyrosinase inhibition and bioavailability potential. Molecules 2020, 25, 2203. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks Jr, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Safety assessment of Vitis vinifera (Grape)-derived ingredients as used in cosmetics. Int. J. Toxicol. 2014, 33, 48S–83S. [Google Scholar] [CrossRef]
- Waffo-Teguo, P.; Lee, D.; Cuendet, M.; Mérillon, J.-M.; Pezzuto, J.M.; Kinghorn, A.D. Two New Stilbene Dimer Glucosides from Grape (Vitis v inifera) Cell Cultures. J. Nat. Prod. 2001, 64, 136–138. [Google Scholar] [CrossRef]
- Baderschneider, B.; Winterhalter, P. Isolation and characterization of novel stilbene derivatives from Riesling wine. J. Agric. Food Chem. 2000, 48, 2681–2686. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Chen, Y. Oligostilbenes from Vitis betulifolia. Phytochemistry 1998, 49, 1393–1394. [Google Scholar] [CrossRef]
- Chiou, W.-F.; Shen, C.-C.; Chen, C.-C.; Lin, C.-H.; Huang, Y.-L. Oligostilbenes from the roots of Vitis thunbergii. Planta Med. 2009, 75, 856–859. [Google Scholar] [CrossRef]
- Lindgren, A.E.G.; Öberg, C.T.; Hillgren, J.M.; Elofsson, M. Total Synthesis of the Resveratrol Oligomers (±)-Ampelopsin B and (±)-ε-Viniferin. Eur. J. Org. Chem. 2016, 2016, 426–429. [Google Scholar] [CrossRef]
- Xue, Y.-Q.; Di, J.-M.; Luo, Y.; Cheng, K.-J.; Wei, X.; Shi, Z. Resveratrol oligomers for the prevention and treatment of cancers. Oxidative Med. Cell. Longev. 2014, 2014, 765832. [Google Scholar] [CrossRef] [Green Version]
- Mikulski, D.; Molski, M. Quantitative structure–antioxidant activity relationship of trans-resveratrol oligomers, trans-4, 4′-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: Trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4′-O-β-D-glucopyranoside. Eur. J. Med. Chem. 2010, 45, 2366–2380. [Google Scholar]
- Cichewicz, R.H.; Kouzi, S.A. Resveratrol oligomers: Structure, chemistry, and biological activity. Stud. Nat. Prod. Chem. 2002, 26, 507–579. [Google Scholar]
- Lins, A.P.; Ribeiro, M.N.D.S.; Gottlieb, O.R.; Gottlieb, H.E. Gnetins: Resveratrol oligomers from Gnetum species. J. Nat. Prod. 1982, 45, 754–761. [Google Scholar] [CrossRef]
- Sotheeswaran, S.; Pasupathy, V. Distribution of resveratrol oligomers in plants. Phytochemistry 1993, 32, 1083–1092. [Google Scholar] [CrossRef]
- Ito, T.; Tanaka, T.; Iinuma, M.; Iliya, I.; Nakaya, K.-i.; Ali, Z.; Takahashi, Y.; Sawa, R.; Shirataki, Y.; Murata, J. New resveratrol oligomers in the stem bark of Vatica pauciflora. Tetrahedron 2003, 59, 5347–5363. [Google Scholar] [CrossRef]
- Abe, N.; Ito, T.; Ohguchi, K.; Nasu, M.; Masuda, Y.; Oyama, M.; Nozawa, Y.; Ito, M.; Iinuma, M. Resveratrol oligomers from Vatica albiramis. J. Nat. Prod. 2010, 73, 1499–1506. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.-N.; Lou, H.-X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef] [PubMed]
- Korhammer, S.; Reniero, F.; Mattivi, F. An oligostilbene from Vitis roots. Phytochemistry 1995, 38, 1501–1504. [Google Scholar] [CrossRef]
- Jean-Denis, J.B.; Pezet, R.; Tabacchi, R. Rapid analysis of stilbenes and derivatives from downy mildew-infected grapevine leaves by liquid chromatography–atmospheric pressure photoionisation mass spectrometry. J. Chromatogr. A 2006, 1112, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Jo, T.H.; Woo, S.S.; Cha, J.; Kim, D.S.; Do, S.; Ryu, J.; Oh, M. Inhibitor for Acetylcholinesterase Containing Gamma-Viniferin or Visitin a. China Patent WO2006025708A2, 2 September 2005. Available online: https://patents.google.com/patent/WO2006025708A2/en (accessed on 2 August 2022).








| Source of Viniferin | Plant Parts | Type of Viniferin | References |
|---|---|---|---|
| Astilbe grandis | Root | α-viniferin | [15] |
| Bombax malabarica | Root bark | ε-viniferin | [16] |
| Caragana chamlagu | Not stated | α-viniferin | [17] |
| Caragana sinica | Root and Stems | α-viniferin | [18,19,20,21] |
| Carex baccans | Not stated | α-viniferin | [22] |
| Carex humilis | Root | α-viniferin | [23,24] |
| Cayratia trifolia | Root | α- and ε-viniferin | [25] |
| Cyphostemma crotalarioides | Root and leaves | cis- and trans-ε-viniferin | [26] |
| Dipterocarpus littoralis | Stem bark | α-viniferin | [27] |
| Dryobalanops lanceolata | Stem bark | ε-viniferin | [28] |
| Shorea roxburghii | Bark and wood parts | α-viniferin | [29] |
| Hopea exalata | Stem bark | α-viniferin | [30] |
| Hopea parviflora | Stem bark | ε-viniferin | [31] |
| Hopea ponga | Stem bark | α-viniferin | [32] |
| Iris clarkei | Seeds | α-viniferin | [33] |
| Iris lactea | Seeds | ε-, R- and R2-viniferin | [34] |
| Rheum undulatum | Not stated | ε- and δ-viniferin | [35] |
| Paeonia lactiflora | Seeds | cis- and trans-ε-viniferin | [36] |
| Paeonia ostii | Seeds | trans-ε-viniferin | [37] |
| Paeonia suffruticosa | Seeds | cis- and trans-ε-viniferin | [38] |
| Parthenocissus quinquefolia | Not stated | ε-viniferin | [39] |
| Rheum lhasaense | Roots | ε-viniferin | [40] |
| Shorea maxwelliana | Stem bark | α-viniferin | [41] |
| Shorea ovalis | Stem bark | α-viniferin | [42] |
| Shorea seminis | Tree bark | α-viniferin | [43] |
| Gnetum microcapum | Not stated | ε-viniferin | [44] |
| Vitis amurensis | Leaves, petioles, berry, skins and seeds | cis- and trans-ε-viniferin | [45,46] |
| Vitis heyneana | Not stated | α-, trans- and R2-viniferin | [47] |
| Vitis labrusca | Not stated | trans ε- and trans δ-viniferin | [48] |
| Vitis quinquangularis | Not stated | α-viniferin | [49] |
| Vitis rotundifolia | Hairy root | ε-viniferin | [50] |
| Vitis thunbergii | Root | ε-viniferin | [51,52,53] |
| Vitis vinifera | Root, stems, canes, leaves, buds and internodes | α-, ε-, ω-, trans, R- and R2-viniferin | [3,54,55,56,57,58,59,60,61] |
| Carbon No. | H (δ), C(δ), J in Hz | |||||
|---|---|---|---|---|---|---|
| Unit I | Unit II | Unit III | ||||
| 2 | 6.07 (br.s) | 86.4 | 5.95 (d, J = 9.7) | 90.0 | 4.90 (d, J = 6.4) | 95.6 |
| 3 | 3.97 (br.s) | 46.4 | 4.71 (d, J = 9.8) | 52.8 | 4.61 (d, J = 6.4) | 55.6 |
| 3a | - | 118.8 | - | 120.9 | - | 119.7 |
| 4 | - | 141.2 | - | 139.7 | - | 138.7 |
| 5 | 5.99 (d, J = 1.8) | 108.5 | 6.72 (d, J = 1.8) | 106.2 | 6.59 (d, J = 1.8) | 105.8 |
| 6 | - | 159.3 | - | 159.3 | - | 160.8 |
| 7 | 6.22 (d, J = 1.8) | 98.0 | 6.25 (d, J = 1.8) | 96.6 | 6.22 (d, J = 1.8) | 96.9 |
| 7a | - | 161.6 | - | 160.6 | - | 161.7 |
| 1′ | - | 132.0 | - | 132.3 | - | 132.5 |
| 2′, 6′ | 7.03 (d, J = 8.5) | 128.2 | 7.22 (d, J = 8.5) | 128.1 | 7.08 (d, J = 8.5) | 128.6 |
| 3′, 5′ | 6.72 (d, J = 8.5) | 115.7 | 6.77 (d, J = 8.5) | 116.1 | 6.79 (d, J = 8.5) | 116.1 |
| 4′ | - | 157.8 | - | 158.2 | - | 158.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuloria, S.; Sekar, M.; Khattulanuar, F.S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Subramaniyan, V.; Jeyabalan, S.; Begum, M.Y.; Chidambaram, K.; et al. Chemistry, Biosynthesis and Pharmacology of Viniferin: Potential Resveratrol-Derived Molecules for New Drug Discovery, Development and Therapy. Molecules 2022, 27, 5072. https://doi.org/10.3390/molecules27165072
Fuloria S, Sekar M, Khattulanuar FS, Gan SH, Rani NNIM, Ravi S, Subramaniyan V, Jeyabalan S, Begum MY, Chidambaram K, et al. Chemistry, Biosynthesis and Pharmacology of Viniferin: Potential Resveratrol-Derived Molecules for New Drug Discovery, Development and Therapy. Molecules. 2022; 27(16):5072. https://doi.org/10.3390/molecules27165072
Chicago/Turabian StyleFuloria, Shivkanya, Mahendran Sekar, Farrah Syazana Khattulanuar, Siew Hua Gan, Nur Najihah Izzati Mat Rani, Subban Ravi, Vetriselvan Subramaniyan, Srikanth Jeyabalan, M. Yasmin Begum, Kumarappan Chidambaram, and et al. 2022. "Chemistry, Biosynthesis and Pharmacology of Viniferin: Potential Resveratrol-Derived Molecules for New Drug Discovery, Development and Therapy" Molecules 27, no. 16: 5072. https://doi.org/10.3390/molecules27165072