Activation of IGF-1/GLP-1 Signalling via 4-Hydroxyisoleucine Prevents Motor Neuron Impairments in Experimental ALS-Rats Exposed to Methylmercury-Induced Neurotoxicity
Abstract
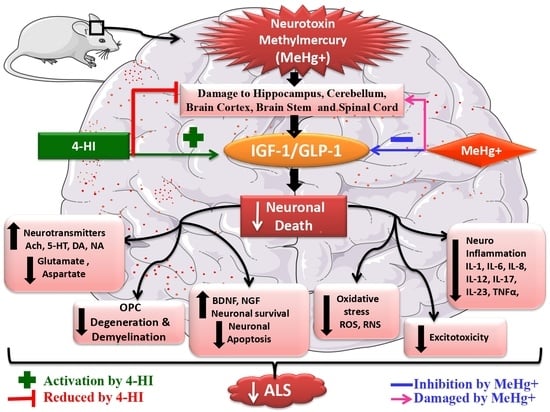
:1. Introduction
2. Material and Methods
2.1. Experimental Animals
2.2. Chemicals and Drugs
2.3. Experimental Animal Model of Methylmercury-Induced ALS-Like Rats
2.4. Protocol Schedule of Animal Experimentation
2.5. Parameters Evaluated
2.5.1. Assessment of Weight Variations
Assessment of Body Weight
Assessment of Relative Brain–Body Weight Ratio
2.6. BehaviourParameters
2.6.1. Grip Strength Test (GST)
2.6.2. Open Field Test (OFT) Assessment
2.6.3. Forced Swim Test (FST)
2.6.4. Morris Water Maze (MWM) Task
2.7. Neurochemical Parameters
2.7.1. Collection and Preparation of Biological Samples
2.7.2. Assessment of Cellular and Molecular Markers
2.7.3. Assessment of Apoptotic Markers
2.7.4. Assessment of Neurotransmitter Levels
2.7.5. Assessment of Inflammatory Cytokine Levels
2.7.6. Assessment of Oxidative Stress Markers
2.7.7. Assessment of Acetylcholinesterase Enzyme Level
2.7.8. Assessment of Gross Pathology and Demyelination Volume in Rat Brain
2.7.9. Assessment of Histopathological Changes
2.8. Statistical Analysis
3. Results
3.1. Effect of 4-Hydroxyisoleucine in the Restoration of Weight Variations after Methyl Mercury-Exposure in Rats
3.1.1. Improved Body Weight after Long-Term Administration of 4-Hydroxyisoleucine
3.1.2. Improvement in Relative Brain–Body Weight Ratio after Long-Term Administration of 4-Hydroxyisoleucine
3.2. Effect of 4-Hydroxyisoleucine in the Amelioration of Neurobehavioral Abnormalities after Methyl Mercury Exposure in Rats
3.2.1. Improved Grip Strength after Long-Term Administration of 4-Hydroxyisoleucine
3.2.2. Improved Locomotion and Restored Anxiety-Like Behaviour after Long-Term Administration of 4-Hydroxyisoleucine
3.2.3. Decreased Depression-Like Behaviour after Long-Term Administration of 4-Hydroxyisoleucine
3.2.4. Improved Memory and Cognition after Long-Term Administration of 4-Hydroxyisoleucine
3.3. Effect of 4-Hydroxyisoleucine on Neurochemical Alterations after Methyl MercuryExposure in Rats
3.3.1. Increased Level of IGF-1 after Long-Term Administration of 4-Hydroxyisoleucine
3.3.2. Increased Level of GLP-1 after Long-Term Administration of 4-Hydroxyisoleucine
3.3.3. Restored Level of Myelin Basic Protein after Long-Term Administration of 4-Hydroxysoleucine
3.3.4. Decreased Levels of Caspase-3 and Bax as well asIncreased Bcl-2 Levels after Long-Term Administration of 4-Hydroxyisoleucine
3.3.5. Restoration of Neurotransmitter Levels after Long-Term Administration of 4-Hydroxyisoleucine
3.3.6. Reduction in Neuroinflammatory Cytokines after Long-Term Administration of 4-Hydroxyisoleucine
3.3.7. Restored Antioxidant Levels after Long-Term Administration of 4-Hydroxyisoleucine
3.4. Effect of 4-HI on Gross Pathological Alterations and Demyelination Volume after Methyl MercuryExposure in Rats
3.4.1. Improvement in Whole-Brain Morphological Alterations after Long-Term Administration of 4-Hydroxyisoleucine
3.4.2. Reduced Pathological Abnormalities in Brain Sections after Long-Term Administration of 4-Hydroxyisoleucine
3.4.3. Reduced Demyelination Volume after Long-Term Administration of 4-Hydroxyisoleucine
3.4.4. Effect of 4-HI onMethyl-Mercury-Induced Histopathological Changes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| AchE | Acetylcholinesterase |
| AkT | Protein kinase B |
| ALS | Amyotrophic lateral sclerosis |
| ALT | Alanine transaminase |
| ANOVA | Analysis of variance |
| AST | Aspartate transaminase |
| Bax | Bcl-2 associated X protein |
| Bcl-2 | B cell lymphoma-2 |
| BDNF | Brain-derived growth factor |
| cAMP | Cyclic AMP |
| Caspase-3 | Cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases-3 |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| ELT | Escape latency time |
| FST | Forced swim test |
| GABA | Gamma amino butyric acid |
| GLP-1 | Glucagon like peptide-1 |
| GLP-1R | Glucagon like peptide-1 receptor |
| GLUT | Glucose transporter |
| GSH | Reduced glutathione |
| GST | Grip strength test |
| HDL | High-density lipoprotein |
| IBD | Intestinal bowel disease |
| IGF-1 | Insulin-like growth factor-1 |
| IGF-1R | Insulin-like growth factor-1 receptor |
| IL-1β | Interleukin-1β |
| IRS-1 | Insulin receptor substrate-1 |
| LDH | Lactate dehydrogenase |
| LDL | Low-density lipoprotein |
| LPS | Lipopolysaccharide |
| MBP | Myelin basic protein |
| MDA | Malondialdehyde |
| MeHg | Methylmercury |
| MND | Motor neuron disease |
| MS | Multiple sclerosis |
| MWM | Morris water maze |
| NGF | Nerve growth factor |
| NO2 | Nitrite |
| ODC | Oligodendrocytes |
| PC12 | Pheochromocytoma cell 12 |
| PI3K | Phosphoinositol 3-kinase |
| rh-IGF-1 | Recombinant human-IGF-1 |
| ROS | Reactive oxygen species |
| SMA | Spontaneous motor activity |
| SOD-1 | Superoxide dismutase 1 |
| TACE | TNF-α converting enzyme |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor-α |
| TSTQ | Time spent in target quadrant |
| 4-HI | 4-hydroxyisoleucine |
| 5-HIAA | 5-hydroxy indole acetic acid |
| 5-HT | Serotonin |
References
- Alam, M.M.; Minj, E.; Yadav, R.K.; Mehan, S. Neuroprotective potential of adenyl cyclase/cAMP/CREB and mitochondrial CoQ10 activator in amyotrophic lateral sclerosis rats. Curr. Bioact. Compd. 2021, 17, 53–69. [Google Scholar] [CrossRef]
- Xia, R.; Liu, Y.; Yang, L.; Gal, J.; Zhu, H.; Jia, J. Motor neuron apoptosis and neuromuscular junction perturbation are prominent features in a Drosophila model of Fus-mediated ALS. Mol. Neurodegener. 2012, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiò, A.; Mazzini, L.; Mora, G. Disease-modifying therapies in amyotrophic lateral sclerosis. Neuropharmacology 2020, 167, 107986. [Google Scholar] [CrossRef] [PubMed]
- Minj, E.; Upadhayay, S.; Mehan, S. Nrf2/HO-1 Signaling Activator Acetyl-11-keto-beta Boswellic Acid (AKBA)-Mediated Neuroprotection in Methyl Mercury-Induced Experimental Model of ALS. Neurochem. Res 2021, 46, 2867–2884. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Wang, Y.; Lu, A.; Hu, C.; Yan, C. DHA ameliorates MeHg-induced PC12 cell apoptosis by inhibiting the ROS/JNK signaling pathway. Mol. Med. Rep 2021, 24, 558. [Google Scholar] [CrossRef]
- Bittencourt, L.O.; Dionizio, A.; Nascimento, P.C.; Puty, B.; Leão, L.K.; Luz, D.A.; Silva, M.C.; Amado, L.L.; Leite, A.; Buzalaf, M.R.; et al. Proteomic approach underlying the hippocampal neurodegeneration caused by low doses of methylmercury after long-term exposure in adult rats. Metallomics 2019, 11, 390–403. [Google Scholar] [CrossRef]
- Zeid, E.H.; Khalifa, B.A.; Said, E.N.; Arisha, A.H.; Reda, R.M. Neurobehavioral and immune-toxic impairments induced by organic methyl mercury dietary exposure in Nile tilapia Oreochromis niloticus. Aquat. Toxicol. 2021, 230, 105702. [Google Scholar] [CrossRef]
- Bassett, T.; Bach, P.; Chan, H.M. Effects of methylmercury on the secretion of pro-inflammatory cytokines from primary microglial cells and astrocytes. Neurotoxicology 2012, 33, 229–234. [Google Scholar] [CrossRef]
- Da Silva Santana, L.N.; Bittencourt, L.O.; Nascimento, P.C.; Fernandes, R.M.; Teixeira, F.B.; Fernandes, L.M.; Silva, M.C.; Nogueira, L.S.; Amado, L.L.; Crespo-Lopez, M.E.; et al. Low doses of methylmercury exposure during adulthood in rats display oxidative stress, neurodegeneration in the motor cortex and lead to impairment of motor skills. J. Trace Elem. Med. Biol. 2019, 51, 19–27. [Google Scholar] [CrossRef]
- Bassil, F.; Fernagut, P.O.; Bezard, E.; Meissner, W.G. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: Targets for disease modification? Prog. Neurobiol. 2014, 118, 1–8. [Google Scholar] [CrossRef]
- Huat, T.J.; Khan, A.A.; Pati, S.; Mustafa, Z.; Abdullah, J.M.; Jaafar, H. IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells. BMC Neurosci. 2014, 15, 91. [Google Scholar] [CrossRef] [Green Version]
- Supeno, N.E.; Pati, S.; Hadi, R.A.; Ghani, A.R.; Mustafa, Z.; Abdullah, J.M.; Idris, F.M.; Han, X.; Jaafar, H. IGF-1 acts as controlling switch for long-term proliferation and maintenance of EGF/FGF-responsive striatal neural stem cells. Int. J. Med. Sci. 2013, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tweedie, D.; Mattson, M.P.; Holloway, H.W.; Greig, N.H. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J. Neurochem. 2010, 113, 1621–1631. [Google Scholar] [CrossRef] [Green Version]
- McMorris, F.A.; Smith, T.M.; DeSalvo, S.; Furlanetto, R.W. Insulin-like growth factor I/somatomedin C: A potent inducer of oligodendrocyte development. Proc. Natl. Acad. Sci. USA 1986, 83, 822–826. [Google Scholar] [CrossRef] [Green Version]
- De Paula, M.L.; Cui, Q.L.; Hossain, S.; Antel, J.; Almazan, G. The PTEN inhibitor bisperoxovanadium enhances myelination by amplifying IGF-1 signaling in rat and human oligodendrocyte progenitors. Glia 2014, 62, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Locatelli, V.; Rizzi, L. Neurotrophic and neuroregenerative effects of GH/IGF1. Int. J. Mol. Sci. 2017, 18, 2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto-Estévez, V.; Defterali, Ç.; Vicario-Abejón, C. IGF-I: A key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shandilya, A.; Mehan, S. IGF-1/GLP-1 Signaling Activator 4-hydroxyisoleucine (4-HI) Prevent Neurobehavioral and Neurochemical Defects in Methylmercury-induced Experimental Model of ALS: Insights From CSF, Blood Plasma and Brain Homogenate Samples in Rats. Res. Sq. 2021. preprint. [Google Scholar]
- Naia, L.; Ferreira, I.L.; Cunha-Oliveira, T.; Duarte, A.I.; Ribeiro, M.; Rosenstock, T.R.; Laço, M.N.; Ribeiro, M.J.; Oliveira, C.R.; Saudou, F.; et al. Activation of IGF-1 and insulin signaling pathways ameliorate mitochondrial function and energy metabolism in Huntington’s Disease human lymphoblasts. Mol. Neurobiol. 2015, 51, 331–348. [Google Scholar] [CrossRef]
- Sun, X.; Huang, L.; Zhang, M.; Sun, S.; Wu, Y. Insulin like growth factor-1 prevents 1-mentyl-4-phenylphyridinium-induced apoptosis in PC12 cells through activation of glycogen synthase kinase-3beta. Toxicology 2010, 271, 5–12. [Google Scholar] [CrossRef]
- Kao, S.Y. Rescue of α-synuclein cytotoxicity by insulin-like growth factors. Biochem. Biophys. Res. Commun. 2009, 385, 434–438. [Google Scholar] [CrossRef]
- Carro, E.; Trejo, J.L.; Gerber, A.; Loetscher, H.; Torrado, J.; Metzger, F.; Torres-Aleman, I. Therapeutic actions of insulin-like growth factor I on APP/PS2 mice with severe brain amyloidosis. Neurobiol. Aging 2006, 27, 1250–1257. [Google Scholar] [CrossRef] [Green Version]
- Park, S.E.; Dantzer, R.; Kelley, K.W.; McCusker, R.H. Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J. Neuroinflammation 2011, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Sun, Z.; Huang, J.; Hu, Y.; Wu, Z.; Mei, B. Mutated recombinant human glucagon-like peptide-1 protects SH-SY5Y cells from apoptosis induced by amyloid-β peptide (1–42). Neurosci. Lett. 2008, 444, 217–221. [Google Scholar] [CrossRef]
- Harkavyi, A.; Abuirmeileh, A.; Lever, R.; Kingsbury, A.E.; Biggs, C.S.; Whitton, P.S. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J. Neuroinflammation 2008, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Perry, T.; Haughey, N.J.; Mattson, M.P.; Egan, J.M.; Greig, N.H. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J. Pharmacol. Exp. Ther. 2002, 302, 881–888. [Google Scholar] [CrossRef] [Green Version]
- Parsarathy, V.; Holscher, C. The novel GLP1 analogue, liraglutide, reduces inflammation in a mouse model of brain tissue injury. In Proceedings of the Society for Neuroscience Annual Meeting, Washington, DC, USA, 12–16 November 2011. [Google Scholar]
- Yoon, G.; Kim, Y.K.; Song, J. Glucagon-like peptide-1 suppresses neuroinflammation and improves neural structure. Pharmacol. Res. 2020, 152, 104615. [Google Scholar] [CrossRef]
- Khera, R.; Mehan, S.; Bhalla, S.; Kumar, S.; Alshammari, A.; Alharbi, M.; Sadhu, S.S. Guggulsterone Mediated JAK/STAT and PPAR-Gamma Modulation Prevents Neurobehavioral and Neurochemical Abnormalities in Propionic Acid-Induced Experimental Model of Autism. Molecules 2022, 27, 889. [Google Scholar] [CrossRef]
- Li, Y.; Duffy, K.B.; Ottinger, M.A.; Ray, B.; Bailey, J.A.; Holloway, H.W.; Tweedie, D.; Perry, T.; Mattson, M.P.; Kapogiannis, D.; et al. GLP-1 receptor stimulation reduces amyloid-β peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J. Alzheimers Dis. 2010, 19, 1205–1219. [Google Scholar] [CrossRef] [Green Version]
- Bomfim, T.R.; Forny-Germano, L.; Sathler, L.B.; Brito-Moreira, J.; Houzel, J.C.; Decker, H.; Silverman, M.A.; Kazi, H.; Melo, H.M.; McClean, P.L.; et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease–associated Aβ oligomers. J. Clin. Investig. 2012, 122, 1339–1353. [Google Scholar] [CrossRef]
- Zafar, M.I.; Gao, F. 4-Hydroxyisoleucine: A potential new treatment for type 2 diabetes mellitus. BioDrugs 2016, 30, 255–262. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; Li, Z.; Liang, Y.; Xu, Q.; Xie, X.; Chen, N. A strategy for L-isoleucine dioxygenase screening and 4-hydroxyisoleucine production by resting cells. Bioengineered 2018, 9, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Maurya, C.K.; Singh, R.; Jaiswal, N.; Venkateswarlu, K.; Narender, T.; Tamrakar, A.K. 4-Hydroxyisoleucine ameliorates fatty acid-induced insulin resistance and inflammatory response in skeletal muscle cells. Mol. Cell. Endocrinol. 2014, 395, 51–60. [Google Scholar] [CrossRef]
- Jaiswal, N.; Maurya, C.K.; Venkateswarlu, K.; Sukanya, P.; Srivastava, A.K.; Narender, T.; Tamrakar, A.K. 4-Hydroxyisoleucine stimulates glucose uptake by increasing surface GLUT4 level in skeletal muscle cells via phosphatidylinositol-3-kinase-dependent pathway. Eur. J. Nutr. 2012, 51, 893–898. [Google Scholar] [CrossRef]
- Haeri, M.R.; Izaddoost, M.; Ardekani, M.R.; Nobar, M.R.; White, K.N. The effect of fenugreek 4-hydroxyisoleucine on liver function biomarkers and glucose in diabetic and fructose-fed rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 61–64. [Google Scholar] [CrossRef]
- Narender, T.; Puri, A.; Khaliq, T.; Saxena, R.; Bhatia, G.; Chandra, R. 4-Hydroxyisoleucine an unusual amino acid as antidyslipidemic and antihyperglycemic agent. Bioorganic Med. Chem. Lett. 2006, 16, 293–296. [Google Scholar] [CrossRef]
- Dayawan, R.; Kaur, S. Studies on the effects of 4-hydroxy isoleucine in experimentally induced inflammatory bowel disease. Int. J. Pharm. Res. 2016, 6, 179–188. [Google Scholar]
- Zhou, C.; Chen, R.; Gao, F.; Zhang, J.; Lu, F. 4-Hydroxyisoleucine relieves inflammation through iRhom2-dependent pathway in co-cultured macrophages and adipocytes with LPS stimulation. BMC Complementary Med. Ther. 2020, 20, 373. [Google Scholar] [CrossRef] [PubMed]
- Avalos-Soriano, A.; la Cruz-Cordero, D.; Rosado, J.L.; Garcia-Gasca, T. 4-Hydroxyisoleucine from fenugreek (Trigonella foenum-graecum): Effects on insulin resistance associated with obesity. Molecules 2016, 21, 1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broca, C.; Breil, V.; Cruciani-Guglielmacci, C.; Manteghetti, M.; Rouault, C.; Derouet, M.; Rizkalla, S.; Pau, B.; Petit, P.; Ribes, G.; et al. Insulinotropic agent ID-1101 (4-hydroxyisoleucine) activates insulin signaling in rat. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E463–E471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, E.M.; Mehan, S.; Upadhayay, S.; Khan, A.; Halawi, M.; Halawi, A.A.; Alsaffar, R.M. Neuroprotective efficacy of 4-Hydroxyisoleucine in experimentally induced intracerebral hemorrhage. Saudi J. Biol. Sci. 2021, 28, 6417–6431. [Google Scholar] [CrossRef]
- Assad, T.; Siddiqui, N.; Rajput, M.A.; Sayyar, H.T.; Soban, M.; Hameed, T. Assessment of antidepressant activity of fenugreek seeds methanol extract. Rawal Med. J. 2021, 46, 236–239. [Google Scholar]
- Kalshetti, P.B.; Alluri, R.; Mohan, V.; Thakurdesai, P.A. Effects of 4-hydroxyisoleucine from fenugreek seeds on depression-like behavior in socially isolated olfactory bulbectomized rats. Pharmacogn. Mag. 2015, 11, S388. [Google Scholar]
- Gaur, V.; Bodhankar, S.L.; Mohan, V.; Thakurdesai, P. Antidepressant-like effect of 4-hydroxyisoleucine from Trigonella foenum graecum L. seeds in mice. Biomed. Aging Pathol. 2012, 2, 121–125. [Google Scholar] [CrossRef]
- Morani, A.S.; Bodhankar, S.L.; Mohan, V.; Thakurdesai, P.A. Ameliorative effects of standardized extract from Trigonella foenum–graecum L. seeds on painful peripheral neuropathy in rats. Asian Pac. J. Trop. Med. 2012, 5, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Jadaun, K.S.; Mehan, S.; Sharma, A.; Siddiqui, E.M.; Kumar, S.; Alsuhaymi, N. Neuroprotective Effect of Chrysophanol as a PI3K/AKT/mTOR Signaling Inhibitor in an Experimental Model of Autologous Blood-induced Intracerebral Hemorrhage. Curr. Med. Sci. 2022. online ahead of print. [Google Scholar] [CrossRef]
- King, K.; Lin, N.P.; Cheng, Y.H.; Chen, G.H.; Chein, R.J. Isolation of positive modulator of glucagon-like peptide-1 signaling from Trigonella foenum-graecum (fenugreek) seed. J. Biol. Chem. 2015, 290, 26235–26248. [Google Scholar] [CrossRef] [Green Version]
- Gautam, S.; Ishrat, N.; Yadav, P.; Singh, R.; Narender, T.; Srivastava, A.K. 4-Hydroxyisoleucine attenuates the inflammation-mediated insulin resistance by the activation of AMPK and suppression of SOCS-3 coimmunoprecipitation with both the IR-β subunit as well as IRS-1. Mol. Cell. Biochem. 2016, 414, 95–104. [Google Scholar] [CrossRef]
- Verma, L.; Sakir, M.; Singh, N.; Mehra, R.; Mehan, S. Development of phase change solutions for ophthalmic drug delivery based on ion activated and pH induced polymers. Int. J. Pharm. Prof. Res. 2010, 1, 127–134. [Google Scholar]
- Rajdev, K.; Siddiqui, E.M.; Jadaun, K.S.; Mehan, S. Neuroprotective potential of solanesol in a combined model of intracerebral and intraventricular hemorrhage in rats. IBRO Rep. 2020, 8, 101–114. [Google Scholar] [CrossRef]
- Sharma, A.; Mehan, S. PI3K/AKT/mTOR Signalling Inhibitor Chrysophanol Ameliorates Neurobehavioural and Neurochemical Defects in Propionic Acid-induced Experimental Model of Autism. Metab. Brain Dis. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Mehan, S.; Rahi, S.; Tiwari, A.; Kapoor, T.; Rajdev, K.; Sharma, R.; Khera, H.; Kosey, S.; Kukkar, U.; Dudi, R. Adenylate cyclase activator forskolin alleviates intracerebroventricular propionic acid-induced mitochondrial dysfunction of autistic rats. Neural Regen. Res. 2020, 15, 1140. [Google Scholar] [CrossRef]
- Sharma, R.; Rahi, S.; Mehan, S. Neuroprotective potential of solanesol in intracerebroventricular propionic acid induced experimental model of autism: Insights from behavioral and biochemical evidence. Toxicol. Rep. 2019, 6, 1164–1175. [Google Scholar] [CrossRef]
- Duggal, P.; Jadaun, K.S.; Siqqiqui, E.M.; Mehan, S. Investigation of low dose cabazitaxel potential as microtubule stabilizer in experimental model of Alzheimer’s disease: Restoring neuronal cytoskeleton. Curr. Alzheimer Res. 2020, 17, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Boynton, F.D.; Dunbar, M.; Koewler, N. General experimental techniques. In The Laboratory Rat; Academic Press: Cambridge, MA, USA, 2020; pp. 771–809. [Google Scholar]
- Gupta, R.; Mehan, S.; Sethi, P.; Prajapati, A.; Alshammari, A.; Alharbi, M.; Al-Mazroua, H.A.; Narula, A.S. Smo-Shh Agonist Purmorphamine Prevents Neurobehavioral and Neurochemical Defects in 8-OH-DPAT-Induced Experimental Model of Obsessive-Compulsive Disorder. Brain Sci. 2022, 12, 342. [Google Scholar] [CrossRef]
- Sahu, R.; Mehan, S.; Kumar, S.; Prajapati, A.; Alshammari, A.; Alharbi, M.; Assiri, M.A.; Narula, A.S. Effect of alpha-mangostin in the prevention of behavioral and neurochemical defects in methylmercury-induced neurotoxicity in experimental rats. Toxicol. Rep. 2022, 9, 977–998. [Google Scholar] [CrossRef]
- Ola, M.S.; Aleisa, A.M.; Al-Rejaie, S.S.; Abuohashish, H.M.; Parmar, M.Y.; Alhomida, A.S.; Ahmed, M.M. Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurol. Sci. 2014, 35, 1003–1008. [Google Scholar] [CrossRef]
- Candeias, E.; Sebastião, I.; Cardoso, S.; Carvalho, C.; Santos, M.S.; Oliveira, C.R.; Moreira, P.I.; Duarte, A.I. Brain GLP-1/IGF-1 signaling and autophagy mediate exendin-4 protection against apoptosis in type 2 diabetic rats. Mol. Neurobiol. 2018, 55, 4030–4050. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Ohta, K.; Nishimura, M.; Saida, T. Detection of myelin basic protein in cerebrospinal fluid and serum from patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Ann. Clin. Biochem. 2002, 39, 603–605. [Google Scholar] [CrossRef]
- Salehi, Z.; Mashayekhi, F.; Naji, M. Insulin like growth factor-1 and insulin like growth factor binding proteins in the cerebrospinal fluid and serum from patients with Alzheimer’s disease. Biofactors 2008, 33, 99–106. [Google Scholar] [CrossRef]
- Rahi, S.; Gupta, R.; Sharma, A.; Mehan, S. Smo-Shh signaling activator purmorphamine ameliorates neurobehavioral, molecular, and morphological alterations in an intracerebroventricular propionic acid-induced experimental model of autism. Hum. Exp. Toxicol. 2021, 40, 1880–1898. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.K.; Mehan, S.; Sahu, R.; Kumar, S.; Khan, A.; Makeen, H.A.; Al Bratty, M. Protective effects of apigenin on methylmercury-induced behavioral/neurochemical abnormalities and neurotoxicity in rats. Hum. Exp. Toxicol. 2022, 41, 09603271221084276. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Khera, R.; Rahi, S.; Mehan, S.; Makeen, H.A.; Khormi, Y.H.; Rehman, M.U.; Khan, A. Neuroprotective Effect of α-Mangostin in Ameliorating Propionic Acid-Induced Experimental Model of Autism in Wistar Rats. Brain Sci. 2021, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Budworth, H.; Snijders, A.M.; Marchetti, F.; Mannion, B.; Bhatnagar, S.; Kwoh, E.; Tan, Y.; Wang, S.X.; Blakely, W.F.; Coleman, M.; et al. DNA repair and cell cycle biomarkers of radiation exposure and inflammation stress in human blood. PLoS ONE 2012, 7, e48619. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Upadhayay, S.; Shandilya, A.; Sahu, R.; Singh, A.; Rajkhowa, B.; Mehan, S. Neuroprotection by solanesol against ethidium bromide-induced multiple sclerosis-like neurobehavioral, molecular, and neurochemical alterations in experimental rats. Phytomedicine Plus 2021, 1, 100051. [Google Scholar] [CrossRef]
- Tao, S.S.; Lu, M.M.; Leng, R.X.; Pan, H.F.; Ye, D.Q. Expression level of plasma Bcl-xL and Bcl-2 in patients with systemic lupus erythematosus. Int. J. Clin. Exp. Med. 2016, 9, 6801–6806. [Google Scholar]
- Alam, M.; Yadav, R.K.; Minj, E.; Tiwari, A.; Mehan, S. Exploring molecular approaches in Amyotrophic lateral sclerosis: Drug targets from clinical and pre-clinical findings. Curr. Mol. Pharmacol. 2021, 14, 263–280. [Google Scholar] [CrossRef]
- Mehan, S.; Monga, V.; Rani, M.; Dudi, R.; Ghimire, K. Neuroprotective effect of solanesol against 3-nitropropionic acid-induced Huntington’s disease-like behavioral, biochemical, and cellular alterations: Restoration of coenzyme-Q10-mediated mitochondrial dysfunction. Indian J. Pharmacol. 2018, 50, 309. [Google Scholar] [CrossRef]
- Khera, H.; Awasthi, A.; Mehan, S. Myocardial preconditioning potential of hedgehog activator purmorphamine (smoothened receptor agonist) against ischemia-reperfusion in deoxycortisone acetate salt-induced hypertensive rat hearts. J. Pharmacol. Pharmacother. 2019, 10, 47. [Google Scholar] [CrossRef]
- Wu, N.; Shen, H.; Liu, H.; Wang, Y.; Bai, Y.; Han, P. Acute blood glucose fluctuation enhances rat aorta endothelial cell apoptosis, oxidative stress and pro-inflammatory cytokine expression in vivo. Cardiovasc. Diabetol. 2016, 15, 109. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Wang, W.; Ding, H.; Yang, Q.; Dong, Q.; Cui, M. The glucagon-like peptide-1 receptor agonist exendin-4 ameliorates warfarin-associated hemorrhagic transformation after cerebral ischemia. J. Neuroinflammation 2016, 13, 204. [Google Scholar] [CrossRef] [Green Version]
- Speaker, K.J.; Cox, S.S.; Paton, M.M.; Serebrakian, A.; Maslanik, T.; Greenwood, B.N.; Fleshner, M. Six weeks of voluntary wheel running modulates inflammatory protein (MCP-1, IL-6, and IL-10) and DAMP (Hsp72) responses to acute stress in white adipose tissue of lean rats. Brain Behav. Immun. 2014, 39, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Mehan, S.; Parveen, S.; Kalra, S. Adenyl cyclase activator forskolin protects against Huntington’s disease-like neurodegenerative disorders. Neural Regen. Res. 2017, 12, 290. [Google Scholar] [CrossRef]
- Kaur, R.; Mehan, S.; Khanna, D.; Kalra, S. Polyphenol Ellagic Acid–Targeting to Brain: A Hidden Treasure. Int. J. Neurol. Res. 2015, 1, 141–152. [Google Scholar]
- Dudi, R.; Mehan, S. Neuroprotection of brain permeable Forskolin ameliorates behavioral, biochemical and histopatho-logical alterations in rat model of intracerebral hemorrhage. Pharmaspire 2018, 10, 68–86. [Google Scholar]
- Singh, L.; Rana, S.; Mehan, S. Role of adenylyl cyclase activator in controlling experimental diabetic nephropathy in rats. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 144. [Google Scholar]
- Deshmukh, R.; Sharma, V.; Mehan, S.; Sharma, N.; Bedi, K.L. Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine—A PDE1 inhibitor. Eur. J. Pharmacol. 2009, 620, 49–56. [Google Scholar] [CrossRef]
- Bala, R.; Khanna, D.; Mehan, S.; Kalra, S. Experimental evidence for the potential of lycopene in the management of scopolamine induced amnesia. RSC Adv. 2015, 5, 72881–72892. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, N.; Khera, R.; Gupta, R.; Mehan, S. Guggulsterone ameliorates ethidium bromide-induced experimental model of multiple sclerosis via restoration of behavioral, molecular, neurochemical and morphological alterations in rat brain. Metab. Brain Dis. 2021, 36, 911–925. [Google Scholar] [CrossRef]
- Praline, J.; Guennoc, A.M.; Limousin, N.; Hallak, H.; de Toffol, B.; Corcia, P. ALS and mercury intoxication: A relationship? Clin. Neurol. Neurosurg. 2007, 109, 880–883. [Google Scholar] [CrossRef]
- Johnson, F.O.; Atchison, W.D. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology 2009, 30, 761–765. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A. Metallomics of Mercury: The Role of Selenium. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2011. [Google Scholar]
- Farina, M.; Rocha, J.B.; Aschner, M. Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci. 2011, 89, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Mori, N.; Yasutake, A.; Marumoto, M.; Hirayama, K. Methylmercury inhibits electron transport chain activity and induces cytochrome c release in cerebellum mitochondria. J. Toxicol. Sci. 2011, 36, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, A.A.; Hort, M.A.; Culbreth, M.; López-Granero, C.; Farina, M.; Rocha, J.B.; Aschner, M. Methylmercury and brain development: A review of recent literature. J. Trace Elem. Med. Biol. 2016, 38, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Sukhanov, S.; Higashi, Y.; Shai, S.Y.; Vaughn, C.; Mohler, J.; Li, Y.; Song, Y.H.; Titterington, J.; Delafontaine, P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2684–2690. [Google Scholar] [CrossRef] [Green Version]
- Chesik, D.; De Keyser, J.; Wilczak, N. Insulin-like growth factor system regulates oligodendroglial cell behavior: Therapeutic potential in CNS. J. Mol. Neurosci. 2008, 35, 81–90. [Google Scholar] [CrossRef]
- Freude, S.; Leeser, U.; Müller, M.; Hettich, M.M.; Udelhoven, M.; Schilbach, K.; Tobe, K.; Kadowaki, T.; Köhler, C.; Schröder, H.; et al. IRS-2 branch of IGF-1 receptor signaling is essential for appropriate timing of myelination. J. Neurochem. 2008, 107, 907–917. [Google Scholar] [CrossRef]
- Shandilya, A.; Mehan, S. Dysregulation of IGF-1/GLP-1 signaling in the progression of ALS: Potential target activators and influences on neurological dysfunctions. Neurol. Sci. 2021, 42, 3145–3166. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk-Marc, I.; Wawrzyniak, A.; Luszczewska-Sierakowska, I.; Babicz, M.A.; Orkisz, S.T. Oligodendrocytes: Morphology, functions and involvement in neurodegenerative diseases. Med. Weter. 2019, 75, 465–471. [Google Scholar] [CrossRef]
- Fields, R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008, 31, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Chiò, A.; Pagani, M.; Agosta, F.; Calvo, A.; Cistaro, A.; Filippi, M. Neuroimaging in amyotrophic lateral sclerosis: Insights into structural and functional changes. Lancet Neurol. 2014, 13, 1228–1240. [Google Scholar] [CrossRef]
- Kang, S.H.; Li, Y.; Fukaya, M.; Lorenzini, I.; Cleveland, D.W.; Ostrow, L.W.; Rothstein, J.D.; Bergles, D.E. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 2013, 16, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorente Pons, A.; Higginbottom, A.; Cooper-Knock, J.; Alrafiah, A.; Alofi, E.; Kirby, J.; Shaw, P.J.; Wood, J.D.; Highley, J.R. Oligodendrocyte pathology exceeds axonal pathology in white matter in human amyotrophic lateral sclerosis. J. Pathol. 2020, 251, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, Z.H. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis 2010, 15, 1382–1402. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Ma, Y.; Guan, F.; Zhang, X.; Chen, W.; Zhang, L.; Zhang, L. Ablation of C9orf72 together with excitotoxicity induces ALS in rats. FEBS J. 2021, 288, 1712–1723. [Google Scholar] [CrossRef]
- Gyawali, A.; Kang, Y.S. Transport alteration of 4-phenyl butyric acid mediated by a sodium-and proton-coupled monocarboxylic acid transporter system in ALS model cell lines (NSC-34) under inflammatory states. J. Pharm. Sci. 2021, 110, 1374–1384. [Google Scholar] [CrossRef]
- Park, E.; Velumian, A.A.; Fehlings, M.G. The role of excitotoxicity in secondary mechanisms of spinal cord injury: A review with an emphasis on the implications for white matter degeneration. J. Neurotrauma 2004, 21, 754–774. [Google Scholar] [CrossRef]
- Foerster, B.R.; Pomper, M.G.; Callaghan, B.C.; Petrou, M.; Edden, R.A.; Mohamed, M.A.; Welsh, R.C.; Carlos, R.C.; Barker, P.B.; Feldman, E.L. An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by use of 3-T proton magnetic resonance spectroscopy. JAMA Neurol. 2013, 70, 1009–1016. [Google Scholar] [CrossRef]
- Lu, C.H.; Allen, K.; Oei, F.; Leoni, E.; Kuhle, J.; Tree, T.; Fratta, P.; Sharma, N.; Sidle, K.; Howard, R.; et al. Systemic inflammatory response and neuromuscular involvement in amyotrophic lateral sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e244. [Google Scholar] [CrossRef] [Green Version]
- Babu, G.N.; Kumar, A.; Chandra, R.; Puri, S.K.; Kalita, J.; Misra, U.K. Elevated inflammatory markers in a group of amyotrophic lateral sclerosis patients from northern India. Neurochem. Res. 2008, 33, 1145–1149. [Google Scholar] [CrossRef]
- Iwai-Shimada, M.; Takahashi, T.; Kim, M.S.; Fujimura, M.; Ito, H.; Toyama, T.; Naganuma, A.; Hwang, G.W. Methylmercury induces the expression of TNF-α selectively in the brain of mice. Sci. Rep. 2016, 6, 38294. [Google Scholar] [CrossRef] [Green Version]
- Shanker, G.; Aschner, J.L.; Syversen, T.; Aschner, M. Free radical formation in cerebral cortical astrocytes in culture induced by methylmercury. Mol. Brain Res. 2004, 128, 48–57. [Google Scholar] [CrossRef]
- Dangoumau, A.; Marouillat, S.; Coelho, R.; Wurmser, F.; Brulard, C.; Haouari, S.; Laumonnier, F.; Corcia, P.; Andres, C.R.; Blasco, H.; et al. Dysregulations of expression of genes of the ubiquitin/sumo pathways in an in vitro model of amyotrophic lateral sclerosis combining oxidative stress and sod1 gene mutation. Int. J. Mol. Sci. 2021, 22, 1796. [Google Scholar] [CrossRef]
- Zuo, X.; Zhou, J.; Li, Y.; Wu, K.; Chen, Z.; Luo, Z.; Zhang, X.; Liang, Y.; Esteban, M.A.; Zhou, Y.; et al. TDP-43 aggregation induced by oxidative stress causes global mitochondrial imbalance in ALS. Nat. Struct. Mol. Biol. 2021, 28, 132–142. [Google Scholar] [CrossRef]
- Saberi, S.; Stauffer, J.E.; Schulte, D.J.; Ravits, J. Neuropathology of amyotrophic lateral sclerosis and its variants. Neurol. Clin. 2015, 33, 855–876. [Google Scholar] [CrossRef] [Green Version]
- Mezzapesa, D.M.; Ceccarelli, A.; Dicuonzo, F.; Carella, A.; De Caro, M.F.; Lopez, M.; Samarelli, V.; Livrea, P.; Simone, I.L. Whole-brain and regional brain atrophy in amyotrophic lateral sclerosis. Am. J. Neuroradiol. 2007, 28, 255–259. [Google Scholar]
- Abrahams, S.; Goldstein, L.H.; Suckling, J.; Ng, V.; Simmons, A.; Chitnis, X.; Atkins, L.; Williams, S.C.; Leigh, P. Frontotemporal white matter changes in amyotrophic lateral sclerosis. J. Neurol. 2005, 252, 321–331. [Google Scholar] [CrossRef]


















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shandilya, A.; Mehan, S.; Kumar, S.; Sethi, P.; Narula, A.S.; Alshammari, A.; Alharbi, M.; Alasmari, A.F. Activation of IGF-1/GLP-1 Signalling via 4-Hydroxyisoleucine Prevents Motor Neuron Impairments in Experimental ALS-Rats Exposed to Methylmercury-Induced Neurotoxicity. Molecules 2022, 27, 3878. https://doi.org/10.3390/molecules27123878
Shandilya A, Mehan S, Kumar S, Sethi P, Narula AS, Alshammari A, Alharbi M, Alasmari AF. Activation of IGF-1/GLP-1 Signalling via 4-Hydroxyisoleucine Prevents Motor Neuron Impairments in Experimental ALS-Rats Exposed to Methylmercury-Induced Neurotoxicity. Molecules. 2022; 27(12):3878. https://doi.org/10.3390/molecules27123878
Chicago/Turabian StyleShandilya, Ambika, Sidharth Mehan, Sumit Kumar, Pranshul Sethi, Acharan S. Narula, Abdulrahman Alshammari, Metab Alharbi, and Abdullah F. Alasmari. 2022. "Activation of IGF-1/GLP-1 Signalling via 4-Hydroxyisoleucine Prevents Motor Neuron Impairments in Experimental ALS-Rats Exposed to Methylmercury-Induced Neurotoxicity" Molecules 27, no. 12: 3878. https://doi.org/10.3390/molecules27123878