Natural and Synthetic Agents Targeting Reactive Carbonyl Species against Metabolic Syndrome
Abstract
:1. Introduction
2. General Overview of Reactive Carbonyl Species (RCS)
3. The Role of RCS in Pathogenesis of MeS and MeS Related Disorders
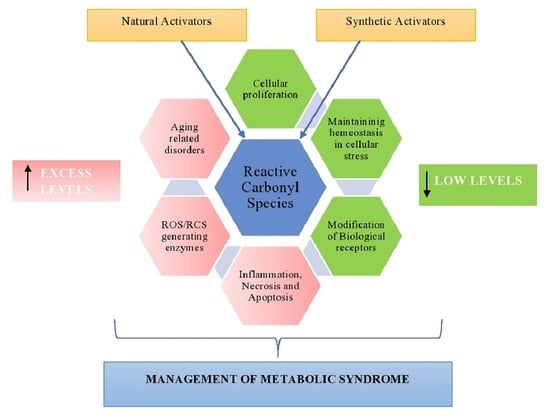
4. Management of Metabolic Disorders Using RCS
4.1. Synthetic Agents Targeting Carbonyl Species for Combatting MeS
4.2. Carnosine
4.3. Histidine Dipeptides
4.4. Metformin
4.5. Simvastatin
4.6. Glycyrrhizin
4.7. Candesartan
5. Natural Agents Targeting Carbonyl Species for Combating MeS
5.1. Natural Products Combatting MeS
5.2. Diabetes
5.2.1. Polyphenols
5.2.2. Nrf-2 Activators
5.2.3. Alpha-Lipoic Acid (ALA)
5.2.4. Melatonin
5.3. Obesity
5.3.1. Terpenoids
5.3.2. Organosulfur
5.3.3. Omega-3 Fatty Acids
5.4. Dyslipidemia
Lipid-Lowering Plants
5.5. Osteoporosis
5.5.1. Lycopenes
5.5.2. Phyto-Estrogens
5.6. Other Disorders
5.6.1. Chromium
5.6.2. Cinnamon
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| AGE | Advanced glycation end products |
| ALE | Advanced Lipoxidation end products |
| APO | Apolipoprotein |
| BMI | Body Mass Index |
| CAT | Chloramphenicol acetyltransferase |
| CNDP 1 | Carnosine Dipeptidase 1 |
| CYP450 | Cytochrome P450 |
| Cr+3 | Trivalent chromium |
| CVD | Cardiovascular disease |
| DHA | Docosahexaenoic acid |
| DNA | Deoxyribonucleic acid |
| EPA | Eicosapentaenoic acid |
| GABA | Gamma-Aminobutyric acid |
| GLUT 4 | Glucose transporter type-4 |
| GSH | Peroxide Glutathione peroxide |
| HDL-C | High density lipoprotein- Cholesterol |
| HMG CoA | β-Hydroxy β-methyl glutaryl-CoA |
| HNE | 4-Hydroxy-trans-2-NonEnal |
| LDL-C | Low density lipoprotein-Cholesterol |
| LMWCr+3 | Low molecular weight chromium binding |
| LOOH | Lipid peroxides |
| LP | Lipoprotein |
| LP-a | Lipoprotein a |
| MetS | Metabolic syndrome |
| mRNA | Messenger RNA |
| NrF2 | Nuclear factor erythroid 2-related factor 2 |
| NTx | N-elopeptide |
| PPAR | Peroxisome proliferator-activated receptor |
| RCS | Reactive carbonyl species |
| ROS | Reactive oxygen species |
| Tchol | Total cholesterol |
| TGs | Triglycerides |
| TNF | Tumor necrosis factor |
| TZDs | Thiazolidinedione |
| T2D | Type-2 diabetes |
References
- Fujita, T. Insulin resistance and salt-sensitive hypertension in metabolic syndrome. Nephrol. Dial. Transplant. 2007, 22, 3102–3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.B.; Petersen, K.F.; Shulman, G.I. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 2007, 87, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Isomaa, B.; Henricsson, M.; Almgren, P.; Tuomi, T.; Taskinen, M.-R.; Groop, L. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia 2001, 44, 1148–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerkar, D.; Mukherjee, A.; Mehta, B.K.; Banerjee, S. Metabolic syndrome associated complications. Int. J. Pharm. Pharm. Sci. 2015, 7, 22–25. [Google Scholar]
- Tian, C.; Zhen, Z. Reactive carbonyl species: Diabetic complication in the heart and lungs. Trends Endocrinol. Metab. 2019, 30, 546–556. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.J.; Rodbard, H.W.; Fox, K.M.; Grandy, S.; Group, S.S. Self-reported prevalence and awareness of metabolic syndrome: Findings from SHIELD. Int. J. Clin. Pract. 2008, 62, 1168–1176. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. A constellation of complications: The metabolic syndrome. Clin. Cornerstone 2005, 7, 36–45. [Google Scholar] [CrossRef]
- Fujita, T. Mineralocorticoid receptors, salt-sensitive hypertension, and metabolic syndrome. Hypertension 2010, 55, 813–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, G.C.; Cipullo, J.P.; Ciorlia, L.A.S.; Cesarino, C.B.; Vilela-Martin, J.F. Prevalence of metabolic syndrome: Association with risk factors and cardiovascular complications in an urban population. PLoS ONE 2014, 9, e105056. [Google Scholar] [CrossRef] [PubMed]
- Carrier, A. Metabolic syndrome and oxidative stress: A complex relationship. Antioxid. Redox Signal. 2017, 26, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic syndrome is associated with oxidative stress and proinflammatory state. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holvoet, P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh. K Acad. Geneeskd. Belg. 2008, 70, 193–219. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar]
- Dobrian, A.D.; Davies, M.J.; Schriver, S.D.; Lauterio, T.J.; Prewitt, R.L. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension 2001, 37, 554–560. [Google Scholar] [CrossRef] [Green Version]
- Hensley, K.; Floyd, R.A. Reactive oxygen species and protein oxidation in aging: A look back, a look ahead. Arch. Biochem. Biophys. 2002, 397, 377–383. [Google Scholar] [CrossRef]
- Ceconi, C.; Boraso, A.; Cargnoni, A.; Ferrari, R. Oxidative stress in cardiovascular disease: Myth or fact? Arch. Biochem. Biophys. 2003, 420, 217–221. [Google Scholar] [CrossRef]
- Pennathur, S.; Heinecke, J.W. Oxidative stress and endothelial dysfunction in vascular disease. Curr. Diabetes Rep. 2007, 7, 257–264. [Google Scholar] [CrossRef]
- Semchyshyn, H.M. Reactive carbonyl species in vivo: Generation and dual biological effects. Sci. World J. 2014, 2014, 417842. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.W.; Lee, Y.-M.; Aldini, G.; Yeum, K.-J. Targeting reactive carbonyl species with natural sequestering agents. Molecules 2016, 21, 280. [Google Scholar] [CrossRef] [Green Version]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Uchida, K. Role of reactive aldehyde in cardiovascular diseases. Free Radic. Biol. Med. 2000, 28, 1685–1696. [Google Scholar] [CrossRef]
- Zimniak, P. Relationship of electrophilic stress to aging. Free Radic. Biol. Med. 2011, 51, 1087–1105. [Google Scholar] [CrossRef] [Green Version]
- Yadav, U.; Ramana, K.V. Regulation of NF-B-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxid. Med. Cell. Longev. 2013, 2013, 690545. [Google Scholar] [CrossRef] [Green Version]
- Pamplona, R. Advanced lipoxidation end-products. Chem. Biol. Interact. 2011, 192, 14–20. [Google Scholar] [CrossRef]
- Matafome, P.; Rodrigues, T.; Sena, C.; Seiça, R. Methylglyoxal in metabolic disorders: Facts, myths, and promises. Med. Res. Rev. 2017, 37, 368–403. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Voziyan, P.; Brown, K.L.; Chetyrkin, S.; Hudson, B. Site-specific AGE modifications in the extracellular matrix: A role for glyoxal in protein damage in diabetes. Clin. Chem. Lab. Med. 2014, 52, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, N.; Thornalley, P.J. Glycation research in amino acids: A place to call home. Amino Acids 2012, 42, 1087–1096. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Siddiqui, Z.; Rehman, S.; Yasir Khan, M.; Khan, H.; Khanum, S.; Alouffi, S.; Saeed, M. A glycation angle to look into the diabetic vasculopathy: Cause and cure. Curr. Vasc. Pharmacol. 2017, 15, 352–364. [Google Scholar] [CrossRef]
- Thornalley, P.J. Glyoxalase I–structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef]
- Chetyrkin, S.; Mathis, M.; Pedchenko, V.; Sanchez, O.A.; McDonald, W.H.; Hachey, D.L.; Madu, H.; Stec, D.; Hudson, B.; Voziyan, P. Glucose autoxidation induces functional damage to proteins via modification of critical arginine residues. Biochemistry 2011, 50, 6102–6112. [Google Scholar] [CrossRef] [Green Version]
- Lapolla, A.; Traldi, P.; Fedele, D. Importance of measuring products of non-enzymatic glycation of proteins. Clin. Biochem. 2005, 38, 103–115. [Google Scholar] [CrossRef]
- Tessier, F.J. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol. Biol. 2010, 58, 214–219. [Google Scholar] [CrossRef]
- Salazar-Ramiro, A.; Ramírez-Ortega, D.; Perez de la Cruz, V.; Hérnandez-Pedro, N.Y.; González-Esquivel, D.F.; Sotelo, J.; Pineda, B. Role of redox status in development of glioblastoma. Front. Immunol. 2016, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.K.; Winocour, P.; Farrington, K. Oxidative stress in early diabetic nephropathy: Fueling the fire. Nat. Rev. Endocrinol. 2011, 7, 176–184. [Google Scholar] [CrossRef]
- Bierhaus, A.; Nawroth, P.P. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 2009, 52, 2251–2263. [Google Scholar] [CrossRef] [Green Version]
- Coughlan, M.T.; Thorburn, D.R.; Penfold, S.A.; Laskowski, A.; Harcourt, B.E.; Sourris, K.C.; Tan, A.L.Y.; Fukami, K.; Thallas-Bonke, V.; Nawroth, P.P. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 2009, 20, 742–752. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, N.; Thornalley, P.J. The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy. Diabetes 2014, 63, 50–52. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp. Gerontol. 2011, 46, 217–224. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2006, 10, 389–406. [Google Scholar] [CrossRef]
- Aldini, G.; de Courten, B.; Regazzoni, L.; Gilardoni, E.; Ferrario, G.; Baron, G.; Altomare, A.; D’Amato, A.; Vistoli, G.; Carini, M. Understanding the antioxidant and carbonyl sequestering activity of carnosine: Direct and indirect mechanisms. Free Radic. Res. 2021, 55, 321–330. [Google Scholar] [CrossRef]
- Taylor, L.E.; Gillis, E.E.; Musall, J.B.; Baban, B.; Sullivan, J.C. High-fat diet-induced hypertension is associated with a proinflammatory T cell profile in male and female Dahl salt-sensitive rats. Am. J. Physiol. Circ. Physiol. 2018, 315, H1713–H1723. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Nishino, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Glucagon-like peptide–1 suppresses advanced glycation end product–induced monocyte chemoattractant protein–1 expression in mesangial cells by reducing advanced glycation end product receptor level. Metabolism 2011, 60, 1271–1277. [Google Scholar] [CrossRef]
- Shao, C.H.; Capek, H.L.; Patel, K.P.; Wang, M.; Tang, K.; DeSouza, C.; Nagai, R.; Mayhan, W.; Periasamy, M.; Bidasee, K.R. Carbonylation contributes to SERCA2a activity loss and diastolic dysfunction in a rat model of type 1 diabetes. Diabetes 2011, 60, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Beisswenger, P.J.; Howell, S.K.; Touchette, A.D.; Lal, S.; Szwergold, B.S. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 1999, 48, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Cuccurullo, C.; Iezzi, A.; Fazia, M.L.; De Cesare, D.; Di Francesco, A.; Muraro, R.; Bei, R.; Ucchino, S.; Spigonardo, F.; Chiarelli, F. Suppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2716–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sil, R.; Ray, D.; Chakraborti, A.S. Glycyrrhizin ameliorates metabolic syndrome-induced liver damage in experimental rat model. Mol. Cell. Biochem. 2015, 409, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Croze, M.L.; Vella, R.E.; Soulère, L.; Lagarde, M.; Soulage, C.O. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology 2012, 153, 2099–2111. [Google Scholar] [CrossRef]
- Figarola, J.L.; Singhal, J.; Rahbar, S.; Awasthi, S.; Singhal, S.S. LR-90 prevents methylglyoxal-induced oxidative stress and apoptosis in human endothelial cells. Apoptosis 2014, 19, 776–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Mori, T.; Guo, Q.; Hu, C.; Ohsaki, Y.; Yoneki, Y.; Zhu, W.; Jiang, Y.; Endo, S.; Nakayama, K. Carbonyl stress induces hypertension and cardio–renal vascular injury in Dahl salt-sensitive rats. Hypertens. Res. 2013, 36, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Gasparotto, J.; Somensi, N.; Bortolin, R.C.; Girardi, C.S.; Kunzler, A.; Rabelo, T.K.; Schnorr, C.E.; Moresco, K.S.; Bassani, V.L.; Yatsu, F.K.J. Preventive supplementation with fresh and preserved peach attenuates CCl4-induced oxidative stress, inflammation and tissue damage. J. Nutr. Biochem. 2014, 25, 1282–1295. [Google Scholar] [CrossRef] [Green Version]
- Semchyshyn, H. Is carbonyl/AGE/RAGE stress a hallmark of the brain aging? Pflüg. Arch. J. Physiol. 2021, 473, 723–734. [Google Scholar] [CrossRef]
- Siems, W.; Grune, T. Intracellular metabolism of 4-hydroxynonenal. Mol. Asp. Med. 2003, 24, 167–175. [Google Scholar] [CrossRef]
- Mano, J.; Kanameda, S.; Kuramitsu, R.; Matsuura, N.; Yamauchi, Y. Detoxification of reactive carbonyl species by glutathione transferase tau isozymes. Front. Plant Sci. 2019, 10, 487. [Google Scholar] [CrossRef] [Green Version]
- Estey, T.; Piatigorsky, J.; Lassen, N.; Vasiliou, V. ALDH3A1: A corneal crystallin with diverse functions. Exp. Eye Res. 2007, 84, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ge, W.; Chen, W.; Kong, X.; Jian, W.; Wang, A. Association Between ALDH2 Gene Polymorphism and Late-onset Alzheimer Disease: An Up-to-Date Meta-Analysis. Curr. Alzheimer Res. 2020, 17, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Amunom, I.; Stephens, L.J.; Conklin, D.J.; Srivastava, S.; Bhatnagar, A.; Prough, R.A. Several Cytochromes P450 Are Aldehyde Monooxygenases. In Proceedings of the 12th Meeting on Enzymology and Molecular Biology of Carbonyl Metabolism Conference, Burlington, VT, USA, 6–11 July 2004; Purdue University Press: Lafayette, IN, USA, 2006; pp. 118–123. [Google Scholar]
- Guo, Y.; Guo, C.; Ha, W.; Ding, Z. Carnosine improves diabetic retinopathy via the MAPK/ERK pathway. Exp. Ther. Med. 2019, 17, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Subramaniyan, V.; Karupiah, S.; Kumari, U.; Sathasivam, K.; Meenakshi, D.U.; Wu, Y.S.; Guad, R.M.; Udupa, K.; Fuloria, N.K. A Comprehensive Review on Source, Types, Effects, Nanotechnology, Detection, and Therapeutic Management of Reactive Carbonyl Species Associated with Various Chronic Diseases. Antioxidants 2020, 9, 1075. [Google Scholar] [CrossRef]
- Brame, C.J.; Salomon, R.G.; Morrow, J.D.; Roberts, L.J. Identification of extremely reactive γ-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J. Biol. Chem. 1999, 274, 13139–13146. [Google Scholar] [CrossRef] [Green Version]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A. New concept in nutrition for the maintenance of the aging eye redox regulation and therapeutic treatment of cataract disease; synergism of natural antioxidant imidazole-containing amino acid-based compounds, chaperone, and glutathione boosting agents: A systemic perspective on aging and longevity emerged from studies in humans. Am. J. Ther. 2010, 17, 373–389. [Google Scholar]
- Neuhouser, M.L.; Wassertheil-Smoller, S.; Thomson, C.; Aragaki, A.; Anderson, G.L.; Manson, J.E.; Patterson, R.E.; Rohan, T.E.; Van Horn, L.; Shikany, J.M. Multivitamin use and risk of cancer and cardiovascular disease in the Women’s Health Initiative cohorts. Arch. Intern. Med. 2009, 169, 294–304. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Rao, L.; Yang, C.; Yuan, H.; Gao, T.; Chen, X.; Sun, H.; Xian, M.; Liu, C. Ratiometric fluorescent probe for monitoring endogenous methylglyoxal in living cells and diabetic blood samples. Anal. Chem. 2019, 91, 5646–5653. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Huang, I.-M.; Hwang, L.S.; Ho, C.-T.; Li, S.; Lo, C.-Y. Anthocyanins in blackcurrant effectively prevent the formation of advanced glycation end products by trapping methylglyoxal. J. Funct. Foods 2014, 8, 259–268. [Google Scholar] [CrossRef]
- Anderson, E.J.; Vistoli, G.; Katunga, L.A.; Funai, K.; Regazzoni, L.; Monroe, T.B.; Gilardoni, E.; Cannizzaro, L.; Colzani, M.; De Maddis, D. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J. Clin. Investig. 2018, 128, 5280–5293. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Dalle-Donne, I.; Facino, R.M.; Milzani, A.; Carini, M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med. Res. Rev. 2007, 27, 817–868. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, L.; de Courten, B.; Garzon, D.; Altomare, A.; Marinello, C.; Jakubova, M.; Vallova, S.; Krumpolec, P.; Carini, M.; Ukropec, J. A carnosine intervention study in overweight human volunteers: Bioavailability and reactive carbonyl species sequestering effect. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Baye, E.; Ukropec, J.; de Courten, M.P.J.; Kurdiova, T.; Krumpolec, P.; Fernández-Real, J.-M.; Aldini, G.; Ukropcova, B.; de Courten, B. Carnosine supplementation reduces plasma soluble transferrin receptor in healthy overweight or obese individuals: A pilot randomised trial. Amino Acids 2019, 51, 73–81. [Google Scholar] [CrossRef]
- Yeum, K.-J.; Orioli, M.; Regazzoni, L.; Carini, M.; Rasmussen, H.; Russell, R.M.; Aldini, G. Profiling histidine dipeptides in plasma and urine after ingesting beef, chicken or chicken broth in humans. Amino Acids 2010, 38, 847–858. [Google Scholar] [CrossRef]
- Holmes, S.B.; Hardee, P.S.G.; Mani, R.R. Percutaneous osteosynthesis of the zygomatic buttress. Br. J. Oral Maxillofac. Surg. 2001, 39, 286–288. [Google Scholar] [CrossRef]
- De Courten, B.; Jakubova, M.; De Courten, M.P.J.; Kukurova, I.J.; Vallova, S.; Krumpolec, P.; Valkovic, L.; Kurdiova, T.; Garzon, D.; Barbaresi, S. Effects of carnosine supplementation on glucose metabolism: Pilot clinical trial. Obesity 2016, 24, 1027–1034. [Google Scholar] [CrossRef]
- Al-Sawalha, N.A.; Alshogran, O.Y.; Awawdeh, M.S.; Almomani, B.A. The effects of l-Carnosine on development of metabolic syndrome in rats. Life Sci. 2019, 237, 116905. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Fantauzzi, C.B.; Pugliese, G. L-carnosine and its derivatives as new therapeutic agents for the prevention and treatment of vascular complications of diabetes. Curr. Med. Chem. 2020, 27, 1744–1763. [Google Scholar] [CrossRef]
- Aldini, G.; Carini, M.; Beretta, G.; Bradamante, S.; Facino, R.M. Carnosine is a quencher of 4-hydroxy-nonenal: Through what mechanism of reaction? Biochem. Biophys. Res. Commun. 2002, 298, 699–706. [Google Scholar] [CrossRef]
- Aldini, G.; Granata, P.; Orioli, M.; Santaniello, E.; Carini, M. Detoxification of 4-hydroxynonenal (HNE) in keratinocytes: Characterization of conjugated metabolites by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2003, 38, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Orioli, M.; Rossoni, G.; Savi, F.; Braidotti, P.; Vistoli, G.; Yeum, K.; Negrisoli, G.; Carini, M. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J. Cell. Mol. Med. 2011, 15, 1339–1354. [Google Scholar] [CrossRef]
- Janssen, B.; Hohenadel, D.; Brinkkoetter, P.; Peters, V.; Rind, N.; Fischer, C.; Rychlik, I.; Cerna, M.; Romzova, M.; de Heer, E. Carnosine as a protective factor in diabetic nephropathy: Association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 2005, 54, 2320–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Hsu, C.; Lin, M.; Liu, K.; Yin, M. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005, 513, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Kurata, H.; Fujii, T.; Tsutsui, H.; Katayama, T.; Ohkita, M.; Takaoka, M.; Tsuruoka, N.; Kiso, Y.; Ohno, Y.; Fujisawa, Y. Renoprotective effects of l-carnosine on ischemia/reperfusion-induced renal injury in rats. J. Pharmacol. Exp. Ther. 2006, 319, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Cahill, L.E.; Fontaine-Bisson, B.; El-Sohemy, A. Functional genetic variants of glutathione S-transferase protect against serum ascorbic acid deficiency. Am. J. Clin. Nutr. 2009, 90, 1411–1417. [Google Scholar] [CrossRef]
- Milman, U.; Blum, S.; Shapira, C.; Aronson, D.; Miller-Lotan, R.; Anbinder, Y.; Alshiek, J.; Bennett, L.; Kostenko, M.; Landau, M. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: A prospective double-blinded clinical trial. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 341–347. [Google Scholar] [CrossRef]
- Freedman, B.I.; Hicks, P.J.; Sale, M.M.; Pierson, E.D.; Langefeld, C.D.; Rich, S.S.; Xu, J.; McDonough, C.; Janssen, B.; Yard, B.A. A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol. Dial. Transplant. 2007, 22, 1131–1135. [Google Scholar] [CrossRef]
- Peters, V.; Schmitt, C.P.; Weigand, T.; Klingbeil, K.; Thiel, C.; van den Berg, A.; Calabrese, V.; Nawroth, P.; Fleming, T.; Forsberg, E. Allosteric inhibition of carnosinase (CN1) by inducing a conformational shift. J. Enzyme Inhib. Med. Chem. 2017, 32, 1102–1110. [Google Scholar] [CrossRef]
- Gallant, S.; Semyonova, M.; Yuneva, M. Carnosine as a potential anti-senescence drug. Biochem. C/c Biokhimiia 2000, 65, 866–868. [Google Scholar]
- Hipkiss, A.R. Glycation, ageing and carnosine: Are carnivorous diets beneficial? Mech. Ageing Dev. 2005, 126, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Niijima, A.; Yamano, T.; Otani, H.; Okumra, N.; Tsuruoka, N.; Nakai, M.; Kiso, Y. Possible role of L-carnosine in the regulation of blood glucose through controlling autonomic nerves. Exp. Biol. Med. 2003, 228, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Everaert, I.; Stegen, S.; Artioli, G.G.; Taes, Y.; Roschel, H.; Achten, E.; Otaduy, M.C.; Junior, A.H.L.; Harris, R. Reduced muscle carnosine content in type 2, but not in type 1 diabetic patients. Amino Acids 2012, 43, 21–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauerhöfer, S.; Yuan, G.; Braun, G.S.; Deinzer, M.; Neumaier, M.; Gretz, N.; Floege, J.; Kriz, W.; Van der Woude, F.; Moeller, M.J. L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 2007, 56, 2425–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoi, B.; He, R.-R.; Yang, D.-H.; Li, Y.-F.; Li, X.-D.; Li, W.-X.; Abe, K.; Kurihara, H. Carnosine ameliorates stress-induced glucose metabolism disorder in restrained mice. J. Pharmacol. Sci. 2011, 117, 223–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chez, M.G.; Buchanan, C.P.; Aimonovitch, M.C.; Becker, M.; Schaefer, K.; Black, C.; Komen, J. Double-blind, placebo-controlled study of L-carnosine supplementation in children with autistic spectrum disorders. J. Child Neurol. 2002, 17, 833–837. [Google Scholar] [CrossRef]
- Park, L.; Raman, K.G.; Lee, K.J.; Lu, Y.; Ferran, L.J.; Chow, W.S.; Stern, D.; Schmidt, A.M. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 1998, 4, 1025–1031. [Google Scholar] [CrossRef]
- Rodriguez, E.L.; Tao, P.; Woolfork, A.G.; Li, Z.; Matsuda, R.; Sun, Z.; Hage, D.S. Studies of binding by sulfonylureas with glyoxal-and methylglyoxal-modified albumin by immunoextraction using affinity microcolumns. J. Chromatogr. A 2021, 1638, 461683. [Google Scholar] [CrossRef]
- Davidson, M.B.; Peters, A.L. An overview of metformin in the treatment of type 2 diabetes mellitus. Am. J. Med. 1997, 102, 99–110. [Google Scholar] [CrossRef]
- Goo, A.K.Y.; Carson, D.S.; Bjelajac, A. Metformin: A new treatment option for non-insulin-dependent diabetes mellitus. J. Fam. Pract. 1996, 42, 612–619. [Google Scholar]
- Sena, C.M.; Matafome, P.; Louro, T.; Nunes, E.; Fernandes, R.; Seiça, R.M. Metformin restores endothelial function in aorta of diabetic rats. Br. J. Pharmacol. 2011, 163, 424–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, K.; Nakamura, J.; Li, W.; Kozakae, M.; Watarai, A.; Nakamura, N.; Yasuda, Y.; Nakashima, E.; Naruse, K.; Watabe, K. Metformin prevents methylglyoxal-induced apoptosis of mouse Schwann cells. Biochem. Biophys. Res. Commun. 2007, 357, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, N.; Liu, T.; Luo, W. Clinical effect of simvastatin combined with exercise training in the treatment of stationary chronic obstructive pulmonary disease complicated with metabolic syndrome. Pak. J. Pharm. Sci. 2020, 33, 437–440. [Google Scholar] [PubMed]
- Verma, D.; Hussain, K.; Namiq, K.S.; Firoz, A.; Bouchama, M.; Raza, M.; Haris, M.; Khan, S. Vitiligo: The Association With Metabolic Syndrome and the Role of Simvastatin as an Immunomodulator. Cureus 2021, 13, e14029. [Google Scholar] [CrossRef]
- Silveira, A.A.A.; Dominical, V.M.; Vital, D.M.; Ferreira, W.A., Jr.; Costa, F.T.M.; Werneck, C.C.; Costa, F.F.; Conran, N. Attenuation of TNF-induced neutrophil adhesion by simvastatin is associated with the inhibition of Rho-GTPase activity, p50 activity and morphological changes. Int. Immunopharmacol. 2018, 58, 160–165. [Google Scholar] [CrossRef]
- Fahim, V.F.; Wadie, W.; Shafik, A.N.; Attallah, M.I. Role of simvastatin and insulin in memory protection in a rat model of diabetes mellitus and dementia. Brain Res. Bull. 2019, 144, 21–27. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, J.; Mo, E.; Zhang, H.; Wang, J.; Hu, X.; Zhou, J.; Bai, H.; Tang, G. A versatile ultrafine and super-absorptive H+-modified montmorillonite: Application for metabolic syndrome intervention and gastric mucosal protection. Biomater. Sci. 2020, 8, 3370–3380. [Google Scholar] [CrossRef]
- Sil, R.; Chakraborti, A.S. Oxidative inactivation of liver mitochondria in high fructose diet-induced metabolic syndrome in rats: Effect of glycyrrhizin treatment. Phytother. Res. 2016, 30, 1503–1512. [Google Scholar] [CrossRef]
- Alvi, S.S.; Nabi, R.; Khan, M.; Akhter, F.; Ahmad, S.; Khan, M.S. Glycyrrhizic Acid Scavenges Reactive Carbonyl Species and Attenuates Glycation-Induced Multiple Protein Modification: An In Vitro and In Silico Study. Oxid. Med. Cell. Longev. 2021, 2021, 7086951. [Google Scholar] [CrossRef]
- Rafiq, K.; Nishiyama, A.; Konishi, Y.; Morikawa, T.; Kitabayashi, C.; Kohno, M.; Masaki, T.; Mori, H.; Kobori, H.; Imanishi, M. Regression of glomerular and tubulointerstitial injuries by dietary salt reduction with combination therapy of angiotensin II receptor blocker and calcium channel blocker in Dahl salt-sensitive rats. PLoS ONE 2014, 9, e107853. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, P.M.; Guha, A.; Stilphen, C.A.; Sun, J.; Jin, C. Proton channels and renal hypertensive injury: A key piece of the Dahl salt-sensitive rat puzzle? Am. J. Physiol. Integr. Comp. Physiol. 2016, 310, R679–R690. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Cowley, A.W., Jr.; Ito, S. Molecular mechanisms and therapeutic strategies of chronic renal injury: Physiological role of angiotensin II-induced oxidative stress in renal medulla. J. Pharmacol. Sci. 2006, 100, 601100006. [Google Scholar] [CrossRef] [Green Version]
- Montezano, A.C.; Touyz, R.M. Molecular mechanisms of hypertension—Reactive oxygen species and antioxidants: A basic science update for the clinician. Can. J. Cardiol. 2012, 28, 288–295. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [Green Version]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-M.; Han, S.-I.; Won, Y.-J.; Lee, E.; Park, E.; Hwang, S.-Y.; Yeum, K.-J. Black rice with giant embryo attenuates obesity-associated metabolic disorders in ob/ob mice. J. Agric. Food Chem. 2016, 64, 2492–2497. [Google Scholar] [CrossRef]
- Miller III, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Lawson, K.A.; Wright, M.E.; Subar, A.; Mouw, T.; Hollenbeck, A.; Schatzkin, A.; Leitzmann, M.F. Multivitamin use and risk of prostate cancer in the National Institutes of Health–AARP Diet and Health Study. J. Natl. Cancer Inst. 2007, 99, 754–764. [Google Scholar] [CrossRef] [Green Version]
- Colzani, M.; Criscuolo, A.; De Maddis, D.; Garzon, D.; Yeum, K.-J.; Vistoli, G.; Carini, M.; Aldini, G. A novel high resolution MS approach for the screening of 4-hydroxy-trans-2-nonenal sequestering agents. J. Pharm. Biomed. Anal. 2014, 91, 108–118. [Google Scholar] [CrossRef]
- Blade, C.; Baselga-Escudero, L.; Salvado, M.J.; Arola-Arnal, A. mi RNA s, polyphenols, and chronic disease. Mol. Nutr. Food Res. 2013, 57, 58–70. [Google Scholar] [CrossRef]
- Saeidnia, S.; Abdollahi, M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol. Appl. Pharmacol. 2013, 273, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Mitjavila, M.T.; Moreno, J.J. The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases. Biochem. Pharmacol. 2012, 84, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Tabatabaei-Malazy, O.; Larijani, B. A systematic review of in vitro studies conducted on effect of herbal products on secretion of insulin from Langerhans islets. J. Pharm. Pharm. Sci. 2012, 15, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Bhakkiyalakshmi, E.; Sireesh, D.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. The emerging role of redox-sensitive Nrf2–Keap1 pathway in diabetes. Pharmacol. Res. 2015, 91, 104–114. [Google Scholar] [CrossRef]
- Gupta, A.; Behl, T.; Sehgal, A.; Bhatia, S.; Jaglan, D.; Bungau, S. Therapeutic potential of Nrf-2 pathway in the treatment of diabetic neuropathy and nephropathy. Mol. Biol. Rep. 2021, 48, 2761–2774. [Google Scholar] [CrossRef]
- Hill, A.M.; Buckley, J.D.; Murphy, K.J.; Howe, P.R.C. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am. J. Clin. Nutr. 2007, 85, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Lorente-Cebrian, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martinez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef]
- Ikeda, M.; Nagashima, T.; Nakamura, E.; Kato, R.; Ohshita, M.; Hayashi, M.; Takeno, S. In vivo roles of fatty acid biosynthesis enzymes in biosynthesis of biotin and α-lipoic acid in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2017, 83, e01322-17. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef]
- Gonzalez-Castejon, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef]
- Hasani-Ranjbar, S.; Nayebi, N.; Larijani, B.; Abdollahi, M. A systematic review of the efficacy and safety of Teucrium species; from anti-oxidant to anti-diabetic effects. IJP—Int. J. Pharmacol. 2010, 6, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Adineh, H.; Naderi, M.; Yousefi, M.; Khademi Hamidi, M.; Ahmadifar, E.; Hoseini, S.M. Dietary licorice (Glycyrrhiza glabra) improves growth, lipid metabolism, antioxidant and immune responses, and resistance to crowding stress in common carp, Cyprinus carpio. Aquac. Nutr. 2021, 27, 417–426. [Google Scholar] [CrossRef]
- Momtaz, S.; Abdollahi, M. An update on pharmacology of Satureja species; from antioxidant, antimicrobial, antidiabetes and anti-hyperlipidemic to reproductive stimulation. Int. J. Pharmacol. 2010, 6, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Hasani-Ranjbar, S.; Nayebi, N.; Moradi, L.; Mehri, A.; Larijani, B.; Abdollahi, M. The efficacy and safety of herbal medicines used in the treatment of hyperlipidemia; a systematic review. Curr. Pharm. Des. 2010, 16, 2935–2947. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Nef, S. Soy, phytoestrogens and metabolism: A review. Mol. Cell. Endocrinol. 2009, 304, 30–42. [Google Scholar] [CrossRef]
- AL-Khateeb, A.R.; Shaari, N.M.; Muid, S.A.; Froemming, G.R.A. A critical link between advanced glycation end products, osteoporosis and type 2 diabetes mellitus. Regen. Res. 2018, 6, 1–9. [Google Scholar]
- de Paula, F.J.A.; Rosen, C.J. Obesity, diabetes mellitus and last but not least, osteoporosis. Arq. Bras. Endocrinol. Metabol. 2010, 54, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Burri, B.J.; Burri, B.J.; Chapman, M.H.; Neidlinger, T.R.; Seo, J.S.; Ishida, B.K.; Burri, B.J.; Chapman, M.H.; Neidlinger, T.R.; Seo, J.S. Tangerine tomatoes increase total and tetra-cis-lycopene isomer concentrations more than red tomatoes in healthy adult humans. Int. J. Food Sci. Nutr. 2009, 60, 1–16. [Google Scholar] [CrossRef]
- Depypere, H.T.; Comhaire, F.H. Herbal preparations for the menopause: Beyond isoflavones and black cohosh. Maturitas 2014, 77, 191–194. [Google Scholar] [CrossRef]
- Poluzzi, E.; Piccinni, C.; Raschi, E.; Rampa, A.; Recanatini, M.; De Ponti, F. Phytoestrogens in postmenopause: The state of the art from a chemical, pharmacological and regulatory perspective. Curr. Med. Chem. 2014, 21, 417–436. [Google Scholar] [CrossRef] [Green Version]
- Power, M.; Pratley, R. Alternative and complementary treatments for metabolic syndrome. Curr. Diabetes Rep. 2011, 11, 173–178. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Hosseinzadeh, H. Cinnamon effects on metabolic syndrome: A review based on its mechanisms. Iran. J. Basic Med. Sci. 2016, 19, 1258. [Google Scholar]
- Crawford, P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: A randomized, controlled trial. J. Am. Board Fam. Med. 2009, 22, 507–512. [Google Scholar] [CrossRef]
- Do, J.-R.; Kim, K.-J.; Jo, J.-H.; Kim, Y.-M.; Kim, B.-S.; Kim, H.-K.; Lim, S.-D.; Lee, S.-W. Antimicrobial, antihypertensive and anticancer activities of medicinal herbs. Korean J. Food Sci. Technol. 2005, 37, 206–213. [Google Scholar]
- Graf, B.L.; Raskin, I.; Cefalu, W.T.; Ribnicky, D.M. Plant-derived therapeutics for the treatment of metabolic syndrome. Curr. Opin. Investig. Drugs 2010, 11, 1107. [Google Scholar]
- Shen, Y.; Jia, L.-N.; Honma, N.; Hosono, T.; Ariga, T.; Seki, T. Beneficial effects of cinnamon on the metabolic syndrome, inflammation, and pain, and mechanisms underlying these effects—A review. J. Tradit. Complement. Med. 2012, 2, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; St-Onge, M.-P.; Heshka, S.; Heymsfield, S.B. Lifestyle behaviors associated with lower risk of having the metabolic syndrome. Metabolism 2004, 53, 1503–1511. [Google Scholar] [CrossRef]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015, 6, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Metabolic Disorders | RCS | Targeted Agent | Experimental Model | References |
|---|---|---|---|---|
| In-vitro Study | ||||
| Diabetic nephropathy | RAGE | Glucagon-like peptide | Human mesangial cells | [49] |
| Insulin resistance | AGEs, Protein carbonyls | NAC, AGD, SAM, D3T | Striated Gastrocnemius muscle | [50] |
| Human Study | ||||
| Diabetes | MG | Metformin | Type II diabetes patients | [51] |
| Diabetes complications | RAGE | Simvastatin | Type II diabetes patients | [52] |
| In-vivo Study | ||||
| Liver damage | AGEs, RAGE, protein carbonyls | Glycyrrhiza | Wistar rats fed with high fructose | [53] |
| Dyslipidemia | HNE (4-Hydroxy-trans-2-NonEnal), AGEs | Carnosine | Zucker rats | [54] |
| Diabetic atherosclerosis | RCS, AGEs, ALEs, RAGE | LR-90 | Diabetic rats induced with streptozotocin | [55] |
| Diabetic neuropathy | RAGE | Candesartan | Dahl salt-sensitive rats injected with MG | [56] |
| Liver/renal toxicity | RAGE, protein carbonyls | Prach | Wistar rats injected with CCl4 | [57] |
| Type II Diabetes | RCS, HNE, PUFAs | Carnosine | Fructose fed Wistar rats and HFHS fed GP 4 het mice | [47] |
| Metabolic Diseases | RCS | Targeted Agent | In-Vivo Model | Reference |
|---|---|---|---|---|
| Dyslipidemia | ALEs, AGEs, RCS, HNE | Carnosine | Zucker obese rats | [47] |
| Renal function | ALEs, AGEs, RCS, HNE | Carnosine | Zucker obese rats | [47] |
| Obesity | HNE, RCS, AGE, PUFAs | Carnosine | Fructose-fed rats | [72] |
| Anticancer | AGEs, ALEs, RCS | MGO | Mitochondria malignant cells | [72] |
| MeS | Drug | Model | Result | Reference |
|---|---|---|---|---|
| Obesity | Carnosine | Obese Humans | Significant decline in the percentage of egested adducts followed by a significant elevation of the urinary excretion of carnosine (through urine) | [74] |
| Dyslipidemia | Carnosine | Zucker obese rats | In obese Zucker rats, both L- and D-CAR (D-carnosine) dramatically reduced obesity-related illnesses by preventing the development of dyslipidemia, hypertension, and kidney damage. | [47] |
| Renal Function | Carnosine | Zucker obese rats | In obese Zucker rats, both L- and D-CAR dramatically reduced obesity-related illnesses by preventing the development of dyslipidemia, hypertension, and kidney damage. | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behl, T.; Gupta, A.; Chigurupati, S.; Singh, S.; Sehgal, A.; Badavath, V.N.; Alhowail, A.; Mani, V.; Bhatia, S.; Al-Harrasi, A.; et al. Natural and Synthetic Agents Targeting Reactive Carbonyl Species against Metabolic Syndrome. Molecules 2022, 27, 1583. https://doi.org/10.3390/molecules27051583
Behl T, Gupta A, Chigurupati S, Singh S, Sehgal A, Badavath VN, Alhowail A, Mani V, Bhatia S, Al-Harrasi A, et al. Natural and Synthetic Agents Targeting Reactive Carbonyl Species against Metabolic Syndrome. Molecules. 2022; 27(5):1583. https://doi.org/10.3390/molecules27051583
Chicago/Turabian StyleBehl, Tapan, Amit Gupta, Sridevi Chigurupati, Sukhbir Singh, Aayush Sehgal, Vishnu Nayak Badavath, Ahmad Alhowail, Vasudevan Mani, Saurabh Bhatia, Ahmed Al-Harrasi, and et al. 2022. "Natural and Synthetic Agents Targeting Reactive Carbonyl Species against Metabolic Syndrome" Molecules 27, no. 5: 1583. https://doi.org/10.3390/molecules27051583