Novel Strategies for Solubility and Bioavailability Enhancement of Bufadienolides
Abstract
:1. Introduction
2. Bufadienolides’ Structure–Activity Relationship
3. Pharmacological Activities of Bufadienolides
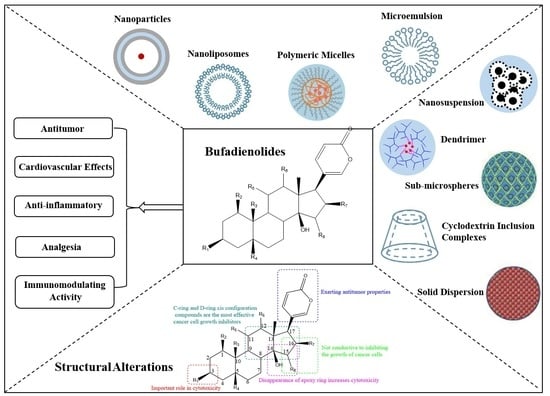
3.1. Antitumor
3.2. Cardiovascular Effects
3.3. Anti-Inflammatory
3.4. Analgesia
3.5. Immunomodulating Activity
| Pharmacological Action | Monomer | Dissolution/Doses | Effects/Mechanisms | Refs. |
|---|---|---|---|---|
| Antitumor | Bufalin | Dissolved in culture medium | Promoted proteasome activation and ATP1A1 protein degradation and thereby inhibited ATP1A1 expression in glioblastoma, inhibiting tumor growth and proliferation. | [20] |
| Dissolved in DMEM | Induced endoplasmic reticulum stress via the IRE1-JNK pathway to inhibit the value added and promoted apoptosis of human hepatoma cell lines Huh-7 and HepG-2. | [51] | ||
| Dissolved in dimethyl sulfoxide (maximum concentration 20 mg/mL) | Suppressed proliferation and induced apoptosis and G2/M phase arrest in pancreatic cancer cells. | [52,53] | ||
| 25, 50, 100 nM | Caused Annexin A2 and DRP1 oligomerization on the surface of mitochondria and disrupted the mitochondrial division/fusion balance to induce U251 cell apoptosis. | [54] | ||
| Had marked antitumor activities by inducing apoptosis. | [55] | |||
| Different concentrations | Reduced the phosphorylation of NOS3, thereby inhibiting the MAPK signaling pathway, and finally suppressed the gastric cancer peritoneal dissemination by inhibiting the EMT process. | [56] | ||
| 10 μM | Had an inhibitory effect on the growth and migration of ovarian cancer cells by inhibiting the activation of mTOR and the induction of HIF1α. | [57] | ||
| Dissolved in DMSO, 1.0 mg/mL | Potentially acted on the Na+/K+ ATPase pump which is overexpressed in melanoma and had the highest anti-proliferative activity on melanoma cells. | [58] | ||
| Diluted in DMSO, 80 nmol/L | Inhibited the proliferation of pancreatic cancer cells, and c-Myc downregulation enhanced this effect. | [59] | ||
| 0.1 mg/kg | Regulated cancer cell stem cells through CD133/NF-κB/MDR1 pathway to reverse colorectal cancer MDR. | [60] | ||
| Cinobufagin | Injection (500 mg/mL) | Showed significant inhibition rates on gastric and hepatocellular tumor growth in vivo. | [61] | |
| 50 μg/mL | Effective inhibition of breast cancer MDAMB-231 cell growth. | [62] | ||
| 0, 50, 100 nM | Induction of apoptosis in osteosarcoma cells via mitochondria-dependent intrinsic apoptotic pathway. | [63] | ||
| Dissolved in 100% DMSO | Selectively suppressed cancer cell viability via DDR-mediated G2 arrest and apoptosis. | [64] | ||
| 0–500 nM | Reduced the proliferation and colony formation of human liver cancer cells in vitro, and induced mitotic arrest of human liver cancer cells. | [26] | ||
| Cinobufotalin | 0.1 μM and 0.2 μM | Inhibited de novo lipogenesis of hepatocellular carcinoma by binding SREBP1 to prevent SREBP1 from sterol regulatory elements and decreasing SREBP1 expression. | [65] | |
| Arenobufagin | Formulated in physiological saline | Inhibited the proliferation of SW1990 and BxPC3 cells and induced cell arrest, apoptosis and autophagy. | [66] | |
| Dissolved in DMSO (10 mM) | Inhibited the proliferation and survival of HER2 overexpressing breast cancer cells. | [30] | ||
| Dissolved in DMSO to a concentration of 500 µM | Showed potent antineoplastic activity against HCC HepG2 cells and corresponding multidrug-resistant HepG2/ADM cells. | [67] | ||
| 10, 20, 40, 100, 150 and 200 ng/mL | Downregulated the expression levels of Cdc25C and Cyclin B1, reduced the cell survival rate. | [22] | ||
| Dissolved in DMSO | Showed selective tumor killing effect on refractory cancer cells. | [23] | ||
| 1β-OH-ABG | At different concentrations | Significantly reduced the expression levels of p-AKT/AKT and p-mTOR (Ser2248 and Ser2481)/mTOR in a time-dependent manner. | [24] | |
| Resibufogenin | Dissolved in DMSO (0.1%) | Inhibited proliferation, migration and invasion of ovarian clear cell carcinomas (OCCC), and induced apoptosis in them. | [68] | |
| Hellebrigenin | 10, 20, 40, 100, 150 and 200 ng/mL | Distinct cytotoxicity against cancerous glial cells with high potency and selectivity. | [22] | |
| 48 nM in SW1990 and 15 nM in BxPC-3 | Inhibited pancreatic cancer cells’ proliferation by inducing cell apoptosis and activation of autophagy via upregulation of apoptosis-related proteins and the autophagic key proteins. | [69] | ||
| Dissolved in DMSO | Induced apoptosis and induced G2/M cell cycle arrest. | [23] | ||
| Marinobufagin Telocinobufagin | 200 mg/mL | Marinobufagin and telocinobufagin have shown remarkable biological action on hematological, solid, sensitive and/or resistant human tumor cell lines. | [25] | |
| ψ-bufarenogin | Dissolved in DMSO | Inhibited the proliferation of liver cancer cells by blocking the cell cycle transition, and downregulated the expression of Mcl-1 to promote cell apoptosis. | [28] | |
| 19-Hydroxybufalin | 10 mM in DMSO | Inhibit the proliferation, migration and invasion of NSCLC cells and promoted the apoptosis of NSCLC cells through the Wnt/β-catenin pathway. | [29] | |
| Cardiovascular effects | Bufalin | 100 mmol/L | Had a biphasic effect on cardiomyocyte contractility. | [38] |
| 10 nM | Suppressed tumor microenvironment-mediated angiogenesis by inhibiting the STAT3 signaling pathway in vascular endothelial cells. | [70] | ||
| Marinobufagin | 0.025, 0.05, and 0.1 nmol·min−1·g body wt−1 | Interacted with the ouabain binding site of the α1Na+-K+-ATPase subunit and thereby influenced cardiac inotropy. | [71] | |
| As a biomarker for preeclampsia. | [72] | |||
| Resibufogenin | 0.2 mg/kg, iv | Induced delayed afterdepolarization and triggered arrhythmias both in cardiac fiber in vitro and in beating heart in vivo at high concentrations. | [39] | |
| 0.3, 1, 3, 10, and 30 μM | Exhibited promising antitumor effect through antiangiogenesis in vivo without obvious toxicity. | [73] | ||
| 1–100 μM | Influenced the cardiac electrical conduction by its multi-channel blocking actions and possessed a proarrhythmic effect at a lower concentration in the working heart of guinea pigs. | [74] | ||
| Arenobufagin | Dissolved in DMSO | Inhibited vascular endothelial growth factor (VEGF)-induced viability, migration, invasion and tube formation in human umbilical vein endothelial cells (HUVECs) in vitro. | [40] | |
| Anti-inflammatory | Bufalin | 100 μL of serum-free medium containing 0, 10, 20, and 30 μM | Suppressed inflammatory cell increase. | [42] |
| Cinobufagin | 50 mg/mL | Significantly decreased the number of proinflammatory factors. | [46] | |
| Gammabufotalin | At nontoxic doses | Inhibition of NF-κB activity exerted anti-inflammatory effects. | [44] | |
| Analgesia | Bufalin | 1, 5 or 20 µM | Inhibited the peak current of nav channels that generate and conduct action potentials in excitable cells. | [49] |
| Cinobufagin | 2 g were soaked in 10× volume of water for injection | Showed stronger analgesic activity and less hepatotoxicity. | [48] | |
| Immunomodulating activity | Bufalin | 20, 50 nM; 50, 100 nM | Directly or indirectly regulated immune response. | [50] |
4. Strategies to Improve Solubility and Bioavailability
4.1. Structural Alterations
4.1.1. Derivative
4.1.2. Prodrug
4.2. Solid Dispersion
4.3. Cyclodextrin Inclusion Complexes
4.4. Nanodelivery Strategies
4.4.1. Nanoparticles
4.4.2. Nanoliposomes
4.4.3. Polymeric Micelles
4.4.4. Microemulsion
Submicroemulsion
Microemulsion
4.4.5. Dendrimer
4.4.6. Nanosuspension
4.4.7. Sub-Microspheres
| Materials | Experimental Subject | Properties | Ref. | |
|---|---|---|---|---|
| Nanoparticles | Bufalin-loaded CaP/DPPE-PEG-EGF | HCT-116 cells, Male BALB/c mice | Showed improved antitumor effects on colon cancer in nude mice, but without severe side effects. | [106] |
| Bufalin-loaded pluronic polyetherimide | HCT116 cells, male athymic nude mice | Had controlled release effect, protected normal tissues from bufalin injury during blood circulation and realized directional and controlled release. | [107] | |
| Bufalin-loaded bovine serum albumin | Kunming mice, nude mice SMMC-7721 cells | Had higher liver uptake and stronger antitumor activity against hepatocellular carcinoma. | [108] | |
| Male Wistar rat | Reduced side effects to a certain extent. | [109] | ||
| Bufalin-loaded mPEG-PLGA-PLL-cRGD | SW620 colon cancer cells, and BALB/c female athymic nude mice | Had good stability, sustained release and tumor targeting, and sustained release in vitro for more than 192 h. | [110] | |
| Bufalin-loaded albumin–polymer hybrid | HepG2 cells, male SD rats | Had good stability, effective tumor targeted delivery potential and side effects reduction ability. | [111] | |
| Gamabufotalin-loaded RBC membrane camouflaged Prussian blue | MDA-MB-231 cells, tumor-bearing BALB/c mice | Prominent in synergistic photothermal/chemotherapy for tumors without side effects on normal tissues. | [116] | |
| Gamabufotalin-loaded GTDC@M-R | MDA-MB-231 cells, BALB/c mice | Showed long blood circulation time, improved bio-safety and accurately accumulated at the tumor site. | [112] | |
| RBG-loaded Gal-SP188-PLGA nanoparticles | HepG2 cells, Kunming mice | Showed excellent in vivo therapeutic effects and anticancer effects, and reduced toxicity. | [113] | |
| Resibufogenin-loaded poly (Lactic-co-glycolic acid)-d-α-tocopheryl polyethylene glycol 1000 succinate | HepG2 cells, Kunming mice | Enhanced the pharmacological effects of liver targeting and reduced the toxicity of RBG. | [114] | |
| Cinobufagin-loaded and folic acid-modified polydopamine | Beas2B, A549, and LLC cell lines, male nude mice | Better therapeutic effect on lung cancer when combined with photothermal therapy. | [115] | |
| Biomimetic nanoparticles loading with gamabufotalin-indomethacin | RAW 264.7 cells, Hela cells, BALB/c mice | Had high biocompatibility and enrichment at the tumor site and reduced side effects of CS-6 on normal cells. | [117] | |
| Nanoliposomes | Liposome-encapsulated BF, CBG and RBG | Improved stability. | [123] | |
| Wistar rats, Kunming mice, HGC-27 and U87-MG cell lines | Reduced side effects, and showed excellent antitumor effects and good blood compatibility. | [124] | ||
| Lovo cells, NCI-H157 cells, SD rats and Kunming mice | Had slow-release properties and better antitumor effect and safety. | [125] | ||
| PEGylated BF211 liposomes | HepG2 cells, BALB/c mice and BALB/c nude mice, pigmented guinea pig, and SD rats | Prolonged blood circulation time, reduced cardiac toxicity, improved tolerance and improved the drug properties. | [126] | |
| Bufalin-loaded PEGylated liposomes | Male SD rats, U251and U87 cells | Improved the solubility and increased the blood concentration of the drug. | [127] | |
| Bufalin liposomes co-modified with transferrin and FA | A549 cells, male BALB/c nude mice | Had the potential to actively deliver drugs to tumor tissues, inhibited tumor growth in mice and had no systemic toxicity. | [128] | |
| Bufalin-loaded wheat germ agglutinin-grafted lipid | Caco-2 cells | Enhanced cell uptake of nanoparticles. | [129] | |
| Showed greater AUC and Cmax, increased oral bioavailability-by 2.7 times. | [130] | |||
| Polymeric Micelles | Bufalin-loaded vitamin E succinate-grafted-chitosan oligosaccharide/rGd conjugated tPGS mixed micelles | HCT116 cells, male SD rats, BALB/c-nu/nu | Exhibited good stability, sustained-release pattern, higher intracellular uptake and greater cytotoxicity. | [131] |
| Bufalin-loaded endosome-escaping polymer and tumor-targeting peptide | HCT116 cells, male SD rats, female BALB/c | Had a better anticancer effect, promoted cell apoptosis and inhibited angiogenesis and anti-proliferation. | [88] | |
| ABG-loaded polymeric nanomicelles | HepG2 cells, male SD rats | Increased the cellular uptake of drug molecules to enhance the anticancer effect of pure drugs. | [133] | |
| DTIs loaded with BF (DTIs-BF) | SMMC-7721 cells | Enhanced the internalization and cytotoxicity of SMMC-7721 cells, and further enhanced the therapeutic effect on SMMC-7721 cells. | [134] | |
| Microemulsion | BF, CBG and RBG-loaded submicron emulsion | ICR mice, BALB/c-nu nude mice, SD rats, HepG2, HCT-8, BGC-803, and EC9706 cells | Had a significant inhibitory effect on HepG2, HCT-8 and EC9706 cells, a slight inhibitory effect on BGC-803 cells in nude mice and no obvious toxicity to mice. | [136] |
| A bufalin self-microemulsifying drug delivery system | Male SD rats | Significantly improved solubility and bioavailability and well absorbed in all intestines. | [139] | |
| Liquid crystalline carriers of bufalin | A549 cells, Wistar rats | 1.4 times enhancement of the cytotoxicity in comparison to the pure BF suspension, increased bioavailability. | [140] | |
| Dendrimer | Bufalin-peptide-dendrimer inclusion through Caco-2 cell monolayer | Caco-2 cells | Improved intestinal permeability and bioavailability. | [146] |
| Nanosuspension | Multicomponent amorphous BF, CBG and RBG nanosuspension | Improved the dissolution performance, and realized the rapid and simultaneous dissolution of multi-component preparations. | [149] | |
| Sub-microspheres | Co-delivery of bufalin and nintedanib via albumin sub-microspheres | HepG2 cellse, male ICR mice, H22 cells | A core-shell structure that enables payload efficiency and stability, good tumor targeting properties, alleviated the tumor microenvironment, exerted a synergistic therapeutic effect. | [152] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume 1, p. 402. [Google Scholar]
- Ying, J.Q.; Yang, M.; Zhang, P.Z.; Zhang, J.L. Research progress on the processing history, chemical composition and pharmacological activity of toad venom. Chin. J. Tradit. Chin. Med. 2021, 46, 3529–3539. [Google Scholar]
- Sun, C.F.; Fan, S.C.; Luo, Y.; Meng, Z.Q.; Xu, J.J. Research progress on chemical constituents and artificial synthesis of Bufonis Venenum. Chin. Tradit. Herb. Drugs 2018, 49, 3183–3192. [Google Scholar]
- Li, F.J.; Hu, J.H.; Ren, X.; Zhou, C.M.; Liu, Q.; Zhang, Y.Q. Toad venom: A comprehensive review of chemical constituents, anticancer activities, and mechanisms. Arch. Pharm. 2021, 354, e2100060. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yu, Z.; Yang, Z.J.; Zhu, G.; Fong, W. Comprehensive chemical analysis of Venenum Bufonis by using liquid chromatography/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, C.; Tian, X.; Huo, X.; Feng, L.; Sun, C.; Ge, G.; Yang, L.; Ning, J.; Ma, X. In vitro phase I metabolism of gamabufotalin and arenobufagin: Reveal the effect of substituent group on metabolic stability. Fitoterapia 2017, 121, 38–45. [Google Scholar] [CrossRef]
- Wu, S.C.; Fu, B.D.; Shen, H.Q.; Yi, P.F.; Zhang, L.Y.; Lv, S.; Guo, X.; Xia, F.; Wu, Y.L.; Wei, X.B. Telocinobufagin enhances the Th1 immune response and protects against Salmonella typhimurium infection. Int. Immunopharmacol. 2015, 25, 353–362. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Wang, X.J.; Ma, J. Research progress of toad venom cardiotoxicity. Chin. J. Pharmacol. Toxicol. 2016, 30, 605–610. [Google Scholar]
- Cao, Y.T.; Cui, K.K.; Wu, J.H.; Pan, H.Y.; Lu, Z.Y.; Shao, J.F.; Wang, L.H. Correlative study of bufogenin constituents in venom of Bufo bufo gargarizans. China J. Chin. Mater. Med. 2019, 44, 1850–1856. [Google Scholar]
- Liu, D.; Feng, J.F. Determination of apparent solubility and apparent oil-water partition coefficient of three toad venom ligand complexes in toad venom. China J. Chin. Mater. Med. 2008, 33, 1256–1258. [Google Scholar]
- Zhong, Y.; Zhao, C.; Wu, W.Y.; Fan, T.Y.; Li, N.G.; Chen, M.; Duan, J.A.; Shi, Z.H. Total synthesis, chemical modification and structure-activity relationship of bufadienolides. Eur. J. Med. Chem. 2020, 189, 112038. [Google Scholar] [CrossRef]
- Yoshiaki, K.; Ayano, Y.; Toshihiko, N.; Hiroshi, M.; Koichi, T.; Hideji, I.; Toshiaki, S.; Ayako, Y.; Kyoko, S.; Mariko, K.; et al. QSAR evaluation of the Ch’an Su and related bufadienolides against the colchicine-resistant primary liver carcinoma cell line PLC/PRF/5(1). J. Med. Chem. 2002, 45, 5440–5447. [Google Scholar]
- Azalim, P.; do Monte, F.M.; Rendeiro, M.M.; Liu, X.; O’Doherty, G.A.; Fontes, C.F.; Leitao, S.G.; Quintas, L.E.M.; Noel, F. Conformational states of the pig kidney Na(+)/K(+)-ATPase differently affect bufadienolides and cardenolides: A directed structure-activity and structure-kinetics study. Biochem. Pharmacol. 2020, 171, 113679. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Xiao, Z.; Ma, B.; Chen, Y.; Liu, M.; Liu, J.; Guo, D.; Liu, X.; Hu, L. Synthesis and biological evaluation of bufalin-3-yl nitrogen-containing-carbamate derivatives as anticancer agents. Steroids 2016, 108, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Y.; Liu, J.H.; Yu, B.Y.; Xu, Q. Microbial transformation of three bufadienolides by Nocardia sp. and some insight for the cytotoxic structure—activity relationship (SAR). Bioorg. Med. Chem. Lett. 2007, 17, 6062–6065. [Google Scholar] [CrossRef]
- Moreno, Y.; Banuls, L.; Urban, E.; Gelbcke, M.; Dufrasne, F.; Kopp, B.; Kiss, R.; Zehl, M. Structure-activity relationship analysis of bufadienolide-induced in vitro growth inhibitory effects on mouse and human cancer cells. J. Nat. Prod. 2013, 76, 1078–1084. [Google Scholar] [CrossRef]
- Shuying, S.; Yi, Z.; Zhen, W.; Rui, L.; Xingguo, G. Bufalin induces the interplay between apoptosis and autophagy in glioma cells through endoplasmic reticulum stress. Int. J. Biol. Sci. 2014, 10, 212–224. [Google Scholar]
- Tsai, S.-C.; Yang, J.-S.; Peng, S.-F.; Lu, C.-C.; Chiang, J.-H.; Chung, J.-G.; Lin, M.-W.; Lin, J.-K.; Amagaya, S.; Chung, C.W.-S.; et al. Bufalin increases sensitivity to AKT/mTOR-induced autophagic cell death in SK-HEP-1 human hepatocellular carcinoma cells. Int. J. Oncol. 2012, 41, 1431–1442. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-Y.; Lu, H.-F.; Hsu, S.-C.; Kuo, C.-L.; Chang, S.-J.; Lin, J.-J.; Wu, P.-P.; Liu, J.-Y.; Lee, C.-H.; Chung, J.-G.; et al. Bufalin inhibits migration and invasion in human hepatocellular carcinoma SK-Hep1 cells through the inhibitions of NF-kB and matrix metalloproteinase-2/-9-signaling pathways. Environ. Toxicol. 2015, 30, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.L.; Wang, X.; Lou, J.C.; Xing, J.S.; Yu, Z.L.; Wang, H.; Zou, S.; Ma, X.; Zhang, B. Bufalin inhibits glioblastoma growth by promoting proteasomal degradation of the Na(+)/K(+)-ATPase alpha1 subunit. Biomed. Pharmacother. 2018, 103, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Nie, L.; Yao, S.; Bi, A.; Ye, Y.; Wu, Y.; Tan, Z.; Wu, Z. Bufalin exerts antitumor effects in neuroblastoma via the induction of reactive oxygen speciesmediated apoptosis by targeting the electron transport chain. Int. J. Mol. Med. 2020, 46, 2137–2149. [Google Scholar] [CrossRef]
- Han, L.; Yuan, B.; Shimada, R.; Hayashi, H.; Si, N.; Zhao, H.Y.; Bian, B.; Takagi, N. Cytocidal effects of arenobufagin and hellebrigenin, two active bufadienolide compounds, against human glioblastoma cell line U-87. Int. J. Oncol. 2018, 53, 2488–2502. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yuan, B.; Bian, B.; Zhao, H.; Kiyomi, A.; Hayashi, H.; Iwatani, Y.; Sugiura, M.; Takagi, N. Cytotoxic Effects of Hellebrigenin and Arenobufagin Against Human Breast Cancer Cells. Front. Oncol. 2021, 11, 711220. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.J.; Lei, Y.H.; Quan, J.Y.; Li, B.J.; Zhang, D.M.; Tian, H.Y.; Chen, Y.; Zhang, E.X.; Chen, L.; Ye, W.C.; et al. 1beta-OH-arenobufagin induces mitochondrial apoptosis in hepatocellular carcinoma through the suppression of mTOR signaling pathway. J. Ethnopharmacol. 2021, 266, 113443. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.Q.; Machado, K.D.; Oliveira, S.F.; Araujo, L.D.; Moncao-Filho, E.D.; Melo-Cavalcante, A.A.; Vieira-Junior, G.M.; Ferreira, P.M. Bufadienolides from amphibians: A promising source of anticancer prototypes for radical innovation, apoptosis triggering and Na+/K+-ATPase inhibition. Toxicon 2017, 127, 63–76. [Google Scholar] [CrossRef]
- Yang, A.L.; Wu, Q.; Hu, Z.D.; Wang, S.P.; Tao, Y.F.; Wang, A.M.; Sun, Y.X.; Li, X.L.; Dai, L.; Zhang, J. A network pharmacology approach to investigate the anticancer mechanism of cinobufagin against hepatocellular carcinoma via downregulation of EGFR-CDK2 signaling. Toxicol. Appl. Pharmacol. 2021, 431, 115739. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H. psi-Bufarenogin, a lead compound of anti-cancer drug. Cell Cycle 2015, 14, 2719–2720. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Wen, W.; Xiang, D.M.; Yin, P.P.; Liu, F.F.; Liu, C.; He, G.P.; Cheng, Z.; Yin, J.P.; Sheng, C.Q.; et al. ψ-Bufarenogin, a novel anti-tumor compound, suppresses liver cancer growth by inhibiting receptor tyrosine kinase-mediated signaling. Oncotarget 2015, 6, 11627–11639. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, X.; Zhang, W.; Xiong, M.; Lin, Y.; Chang, M.; Xu, L.; Lu, Y.; Liu, Y.; Zhang, J. 19-Hydroxybufalin inhibits non-small cell lung cancer cell proliferation and promotes cell apoptosis via the Wnt/beta-catenin pathway. Exp. Hematol. Oncol. 2021, 10, 48. [Google Scholar] [CrossRef]
- Wang, T.; Mu, L.; Jin, H.; Zhang, P.; Wang, Y.; Ma, X.; Pan, J.; Miao, J.; Yuan, Y. The effects of bufadienolides on HER2 overexpressing breast cancer cells. Tumor Biol. 2016, 37, 7155–7163. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Shen, K.; Wang, R.; Chen, C.; Liao, Z.; Zhou, J. Paclitaxel Suppresses Hepatocellular Carcinoma Tumorigenesis Through Regulating Circ-BIRC6/miR-877-5p/YWHAZ Axis. Oncol. Targets Ther. 2020, 13, 9377–9388. [Google Scholar] [CrossRef]
- Zhang, J.H.; Shu, C.Y.; Yi, X.J.; Zhu, J.F.; Lian, X.Y.; Wu, Y.J. Response Surface Methodology to Optimize the Combination Treatment of Paclitaxel, Bufalin and Cinobufagin for Hepatoma Therapy. Comb. Chem. High Throughput Screen. 2020, 24, 1727–1735. [Google Scholar] [CrossRef]
- Gu, R.; Zhang, Q. Effects of low-dose bufalin combined with hydroxycamptothecin on human castration-resistant prostate cancer xenografts in nude mice. Exp. Ther. Med. 2021, 22, 1015. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Xu, K.; Shimada, R.; Li, J.; Hayashi, H.; Okazaki, M.; Takagi, N. Cytotoxic Effects of Arsenite in Combination with Gamabufotalin Against Human Glioblastoma Cell Lines. Front. Oncol. 2021, 11, 628914. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ma, R.; Cao, G.; Liu, H.; He, L.; Tang, L.; Li, H.; Luo, Q. Combined Treatment of Cinobufotalin and Gefitinib Exhibits Potent Efficacy against Lung Cancer. Evid. Based Complement. Altern. Med. 2021, 2021, 6612365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Bian, X.L.; Guo, F.J.; Wu, Y.C.; Li, Y.M. Two new 19-norbufadienolides with cardiotonic activity isolated from the venom of Bufo bufo gargarizans. Fitoterapia 2018, 131, 215–220. [Google Scholar] [CrossRef]
- Wei, W.L.; Hou, J.J.; Wang, X.; Yu, Y.; Li, H.J.; Li, Z.W.; Feng, Z.J.; Qu, H.; Wu, W.Y.; Guo, D.A. Venenum bufonis: An overview of its traditional use, natural product chemistry, pharmacology, pharmacokinetics and toxicology. J. Ethnopharmacol. 2019, 237, 215–235. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.-J.; Zhao, Q.; Wang, J.-X.; Xing, H.-Y.; Zhang, Y.-Z.; Zhang, X.-X.; Zhi, Y.-Y.; Li, H.; Ma, J. Bufalin-induced cardiotoxicity: New findings into mechanisms. Chin. J. Nat. Med. 2020, 18, 550–560. [Google Scholar] [CrossRef]
- Xie, J.T.; Dey, L.; Wu, J.A.; Lowell, T.K.; Yuan, C.S. Cardiac toxicity of resibufogenin: Electrophysiological evidence. Acta Pharmacol. Sin. 2001, 22, 289–297. [Google Scholar]
- Li, M.; Wu, S.; Liu, Z.; Zhang, W.; Xu, J.; Wang, Y.; Liu, J.; Zhang, D.; Tian, H.; Li, Y.; et al. Arenobufagin, a bufadienolide compound from toad venom, inhibits VEGF-mediated angiogenesis through suppression of VEGFR-2 signaling pathway. Biochem. Pharmacol. 2012, 83, 1251–1260. [Google Scholar] [CrossRef]
- Ren, W.; Luo, Z.; Pan, F.; Liu, J.; Sun, Q.; Luo, G.; Wang, R.; Zhao, H.; Bian, B.; Xiao, X.; et al. Integrated network pharmacology and molecular docking approaches to reveal the synergistic mechanism of multiple components in Venenum Bufonis for ameliorating heart failure. PeerJ 2020, 8, e10107. [Google Scholar] [CrossRef]
- Rong, X.; Ni, W.; Liu, Y.; Wen, J.; Qian, C.; Sun, L.; Wang, J. Bufalin, a bioactive component of the Chinese medicine chansu, inhibits inflammation and invasion of human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation 2014, 37, 1050–1058. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; Church, T.D.; Lugogo, N.L.; Que, L.G.; Francisco, D.; Ingram, J.L.; Huggins, M.; Beaver, D.M.; Wright, J.R.; et al. SHP-1 as a critical regulator of Mycoplasma pneumoniae-induced inflammation in human asthmatic airway epithelial cells. J. Immunol. 2012, 188, 3371–3381. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Deng, L.; Cao, H.; Xu, N.; Zhang, D.; Tian, H.; Li, B.; Lu, Z.; Ye, W.; Yu, L.; et al. Screening of Bufadienolides from Toad Venom Identifies Gammabufotalin as a Potential Anti-inflammatory Agent. Planta Med. 2020, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Zhakeer, Z.; Hadeer, M.; Tuerxun, Z.; Tuerxun, K. Bufalin Inhibits the Inflammatory Effects in Asthmatic Mice through the Suppression of Nuclear Factor-Kappa B Activity. Pharmacology 2017, 99, 179–187. [Google Scholar] [CrossRef]
- Wang, S.-W.; Bai, Y.-F.; Weng, Y.-Y.; Fan, X.-Y.; Huang, H.; Zheng, F.; Xu, Y.; Zhang, F. Cinobufacini Ameliorates Dextran Sulfate Sodium–Induced Colitis in Mice through Inhibiting M1 Macrophage Polarization. J. Pharmacol. Exp. Ther. 2019, 368, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Shu-Shu, Q.; Yao-Guo, C.; Dong-Yun, L.; Qian, Y. Systematic review and Meta-analysis of efficacy and safety of Huachansu in treating cancer-related pain. China J. Chin. Mater. Med. 2019, 44, 2627–2636. [Google Scholar]
- Xu, L.; Wang, S.; Shen, H.; Feng, Q.; Zhang, X.; Ni, H.; Yao, M. Analgesic and toxic effects of venenum bufonis and its constituent compound cinobufagin: A comparative study. Neurotoxicol. Teratol. 2019, 73, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Jiang, F.; Liu, C.; Liu, Z.; Zhu, Y.; Xu, J.; Ge, Y.; Xu, K.; Yin, P. Modulatory effects of bufalin, an active ingredient from toad venom on voltage-gated sodium channels. Mol. Biol. Rep. 2018, 45, 721–740. [Google Scholar] [CrossRef]
- Fu, R.; Yu, F.; Wu, W.; Liu, J.; Li, J.; Guo, F.; Xu, L.; Wang, F.; Cui, X. Bufalin enhances the killing efficacy of NK cells against hepatocellular carcinoma by inhibiting MICA shedding. Int. Immunopharmacol. 2021, 101, 108195. [Google Scholar] [CrossRef]
- Hu, F.; Han, J.; Zhai, B.; Ming, X.; Zhuang, L.; Liu, Y.; Pan, S.; Liu, T. Blocking autophagy enhances the apoptosis effect of bufalin on human hepatocellular carcinoma cells through endoplasmic reticulum stress and JNK activation. Apoptosis 2014, 19, 210–223. [Google Scholar] [CrossRef]
- Li, M.; Yu, X.; Guo, H.; Sun, L.; Wang, A.; Liu, Q.; Wang, X.; Li, J. Bufalin exerts antitumor effects by inducing cell cycle arrest and triggering apoptosis in pancreatic cancer cells. Tumor. Biol. 2014, 35, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yin, S.; Li, J.; Jiang, C.; Ye, M.; Hu, H. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis 2011, 16, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Wang, X.; Yang, Q.; Zhou, X.; Wu, J.; Yang, X.; Zhao, Y.; Lin, R.; Xie, Y.; et al. Bufalin induces mitochondrial dysfunction and promotes apoptosis of glioma cells by regulating Annexin A2 and DRP1 protein expression. Cancer Cell Int. 2021, 21, 424. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.-H.; Liu, X.; Qiu, Y.-Y.; Cai, J.-F.; Qin, J.-M.; Zhu, H.-R.; Li, Q. Anti-tumor Activity and Apoptosis-regulation Mechanisms of Bufalin in Various Cancers: New Hope for Cancer Patients. Asian Pac. J. Cancer Prev. 2012, 13, 5339–5343. [Google Scholar] [CrossRef] [Green Version]
- Zou, D.; Song, J.; Deng, M.; Ma, Y.; Yang, C.; Liu, J.; Wang, S.; Wen, Z.; Tang, Y.; Qu, X.; et al. Bufalin inhibits peritoneal dissemination of gastric cancer through endothelial nitric oxide synthase-mitogen-activated protein kinases signaling pathway. FASEB J. 2021, 35, e21601. [Google Scholar] [CrossRef]
- Su, S.; Dou, H.; Wang, Z.; Zhang, Q. Bufalin inhibits ovarian carcinoma via targeting mTOR/HIF-alpha pathway. Basic Clin. Pharm. Toxicol. 2021, 128, 224–233. [Google Scholar] [CrossRef]
- Soumoy, L.; Wells, M.; Najem, A.; Krayem, M.; Ghanem, G.; Hambye, S.; Saussez, S.; Blankert, B.; Journe, F. Toad Venom Antiproliferative Activities on Metastatic Melanoma: Bio-Guided Fractionation and Screening of the Compounds of Two Different Venoms. Biology 2020, 9, 218. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Peng, J.; Xie, B.; Shou, Q.; Wang, J. Silencing c-Myc Enhances the Antitumor Activity of Bufalin by Suppressing the HIF-1alpha/SDF-1/CXCR4 Pathway in Pancreatic Cancer Cells. Front. Pharmacol. 2020, 11, 495. [Google Scholar] [CrossRef]
- Zhan, Y.; Qiu, Y.; Wang, H.; Wang, Z.; Xu, J.; Fan, G.; Xu, J.; Li, W.; Cao, Y.; Le, V.M.; et al. Bufalin reverses multidrug resistance by regulating stemness through the CD133/nuclear factor-kappaB/MDR1 pathway in colorectal cancer. Cancer Sci. 2020, 111, 1619–1630. [Google Scholar] [CrossRef]
- Wei, X.; Si, N.; Zhang, Y.; Zhao, H.; Yang, J.; Wang, H.; Wang, L.; Han, L.; Bian, B. Evaluation of Bufadienolides as the Main Antitumor Components in Cinobufacin Injection for Liver and Gastric Cancer Therapy. PLoS ONE 2017, 12, e0169141. [Google Scholar] [CrossRef]
- Ma, L.; Song, B.; Jin, H.; Pi, J.; Liu, L.; Jiang, J.; Cai, J. Cinobufacini induced MDA-MB-231 cell apoptosis-associated cell cycle arrest and cytoskeleton function. Bioorg. Med. Chem. Lett. 2012, 22, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Zheng, D.; Guo, W.; Yang, J.; Cheng, A.Y. Cinobufagin Induces Apoptosis in Osteosarcoma Cells Via the Mitochondria-Mediated Apoptotic Pathway. Cell Physiol. Biochem. 2018, 46, 1134–1147. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Wang, J.; Zhang, Q.; Zou, Z.; Ding, Y. Cinobufagin-induced DNA damage response activates G2/M checkpoint and apoptosis to cause selective cytotoxicity in cancer cells. Cancer Cell Int. 2021, 21, 446. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Shen, M.; Li, J.; Zhang, R.; Li, X.; Zhao, L.; Huang, G.; Liu, J. Novel SREBP1 inhibitor cinobufotalin suppresses proliferation of hepatocellular carcinoma by targeting lipogenesis. Eur. J. Pharmacol. 2021, 906, 174280. [Google Scholar] [CrossRef]
- Wei, X.; Yang, J.; Mao, Y.; Zhao, H.; Si, N.; Wang, H.; Bian, B. Arenobufagin Inhibits the Phosphatidylinositol 3-kinase/Protein Kinase B/Mammalian Target of Rapamycin Pathway and Induces Apoptosis and Autophagy in Pancreatic Cancer Cells. Pancreas 2020, 49, 261–272. [Google Scholar] [CrossRef]
- Zhang, D.M.; Liu, J.S.; Deng, L.J.; Chen, M.F.; Yiu, A.; Cao, H.H.; Tian, H.Y.; Fung, K.P.; Kurihara, H.; Pan, J.X.; et al. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis 2013, 34, 1331–1342. [Google Scholar] [CrossRef] [Green Version]
- Guannan, Z.; Zhongyi, Z.; Lihua, L.; Jingxin, D. Resibufogenin inhibits ovarian clear cell carcinoma (OCCC) growth in vivo, and migration of OCCC cells in vitro, by down-regulating the PI3K/AKT and actin cytoskeleton signaling pathways. Am. J. Transl. Res. 2019, 11, 6290–6303. [Google Scholar]
- Wei, X.; He, J.; Gao, B.; Han, L.; Mao, Y.; Zhao, H.; Si, N.; Wang, H.; Yang, J.; Bian, B. Hellebrigenin anti-pancreatic cancer effects based on apoptosis and autophage. PeerJ 2020, 8, e9011. [Google Scholar] [CrossRef]
- Fang, K.; Zhan, Y.; Zhu, R.; Wang, Y.; Wu, C.; Sun, M.; Qiu, Y.; Yuan, Z.; Liang, X.; Yin, P.; et al. Bufalin suppresses tumour microenvironment-mediated angiogenesis by inhibiting the STAT3 signalling pathway. J. Transl. Med. 2021, 19, 383. [Google Scholar] [CrossRef]
- Wansapura, A.N.; Lasko, V.; Xie, Z.; Fedorova, O.V.; Bagrov, A.Y.; Lingrel, J.B.; Lorenz, J.N. Marinobufagenin enhances cardiac contractility in mice with ouabain-sensitive alpha1 Na+-K+-ATPase. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1833–H1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenaerts, C.; Demeyer, M.; Gerbaux, P.; Blankert, B. Analytical aspects of marinobufagenin. Clin. Chim. Acta 2013, 421, 193–201. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, Y.X.; Wu, Y.; Lu, D.; Huang, R.; Wang, L.L.; Wang, S.Q.; Guan, Y.Y.; Zhang, H.; Luan, X. Resibufogenin Suppresses Triple-Negative Breast Cancer Angiogenesis by Blocking VEGFR2-Mediated Signaling Pathway. Front. Pharmacol. 2021, 12, 682735. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Li, J.; Wang, M.; Su, M.; Xu, D.; Zhou, L.; Zhang, X.; Wang, H.; Hou, Y. Analysis of Resibufogenin on Cardiac conduction reveals a species difference in the cardiac electrophysiology: Rats versus guinea pigs. Biomed. Pharmacother. 2021, 139, 111581. [Google Scholar] [CrossRef] [PubMed]
- Di, L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H.; Carter, G.T. Drug-Like Property Concepts in Pharmaceutical Design. Curr. Pharm. Des. 2009, 15, 2184–2194. [Google Scholar] [CrossRef]
- Xiao, D.; Liu, Z.; Zhang, S.; Zhou, M.; He, F.; Zou, M.; Peng, J.; Xie, X.; Liu, Y.; Peng, D. Berberine Derivatives with Different Pharmacological Activities via Structural Modifications. Mini-Rev. Med. Chem. 2018, 18, 1424–1441. [Google Scholar] [CrossRef]
- Liu, J.S.; Deng, L.J.; Tian, H.Y.; Ruan, Z.X.; Cao, H.H.; Ye, W.C.; Zhang, D.M.; Yu, Z.L. Anti-tumor effects and 3D-quantitative structure-activity relationship analysis of bufadienolides from toad venom. Fitoterapia 2019, 134, 362–371. [Google Scholar] [CrossRef]
- Wu, X.Y.; Tian, F.; Su, M.H.; Wu, M.; Huang, Y.; Hu, L.H.; Jin, L.; Zhu, X.J. BF211, a derivative of bufalin, enhances the cytocidal effects in multiple myeloma cells by inhibiting the IL-6/JAK2/STAT3 pathway. Int. Immunopharmacol. 2018, 64, 24–32. [Google Scholar] [CrossRef]
- Liu, M.; Feng, L.X.; Sun, P.; Liu, W.; Wu, W.Y.; Jiang, B.H.; Yang, M.; Hu, L.H.; Guo, D.A.; Liu, X. A Novel Bufalin Derivative Exhibited Stronger Apoptosis-Inducing Effect than Bufalin in A549 Lung Cancer Cells and Lower Acute Toxicity in Mice. PLoS ONE 2016, 11, e0159789. [Google Scholar] [CrossRef]
- Ma, B.; Xiao, Z.Y.; Chen, Y.J.; Lei, M.; Meng, Y.H.; Guo, D.A.; Liu, X.; Hu, L.H. Synthesis and structure—Activity relationships study of cytotoxic bufalin 3-nitrogen-containing-ester derivatives. Steroids 2013, 78, 508–512. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, J.; Long, H.; Zhang, Z.; Lei, M.; Wu, W. Design, synthesis and anti-tumor activities of carbamate derivatives of cinobufagin. Steroids 2020, 164, 108749. [Google Scholar] [CrossRef]
- Chen, B.; Wang, C.; Ma, J.; Ma, H.; Wang, Y.; Zhang, H.; Zhu, Y.; Yao, J.; Luo, C.; Miao, Z.; et al. Discovery of 3-peptide substituted arenobufagin derivatives as potent antitumor agents with low cardiotoxicity. Steroids 2021, 166, 108772. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, C.; Zhao, M.; Chen, H.; Liu, X.; Chen, J.; Lonard, D.M.; Qin, L.; Xu, J.; Wang, X.; et al. Steroid Receptor Coactivator-3 (SRC-3/AIB1) as a Novel Therapeutic Target in Triple Negative Breast Cancer and Its Inhibition with a Phospho-Bufalin Prodrug. PLoS ONE 2015, 10, e0140011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Xu, W.; Huang, Z.H.; Guo, J.; Jiang, R.W. An Efficient Strategy for the Chemo-Enzymatic Synthesis of Bufalin Glycosides with Improved Water Solubility and Inhibition against Na+, K+-ATPase. Chem. Biodivers. 2020, 17, e2000529. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yuan, X.; Jia, T.; Liu, C.; Ni, Z.; Qin, Z.; Yuan, Y. Polymeric prodrug of bufalin for increasing solubility and stability: Synthesis and anticancer study in vitro and in vivo. Int. J. Pharm. 2016, 506, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jia, T.; Yuan, X.; Liu, C.; Sun, J.; Ni, Z.; Xu, J.; Wang, X.; Yuan, Y. Development of octreotide-conjugated polymeric prodrug of bufalin for targeted delivery to somatostatin receptor 2 overexpressing breast cancer in vitro and in vivo. Int. J. Nanomed. 2016, 11, 2235–2250. [Google Scholar]
- Shi, X.-J.; Qiu, Y.-Y.; Yu, H.; Liu, C.; Yuan, Y.-X.; Yin, P.-H.; Liu, T. Increasing the anticancer performance of bufalin (BUF) by introducing an endosome-escaping polymer and tumor-targeting peptide in the design of a polymeric prodrug. Colloids Surf. B Biointerfaces 2018, 166, 224–234. [Google Scholar] [CrossRef]
- Deng, L.J.; Wang, L.H.; Peng, C.K.; Li, Y.B.; Huang, M.H.; Chen, M.F.; Lei, X.P.; Qi, M.; Cen, Y.; Ye, W.C.; et al. Fibroblast Activation Protein alpha Activated Tripeptide Bufadienolide Antitumor Prodrug with Reduced Cardiotoxicity. J. Med. Chem. 2017, 60, 5320–5333. [Google Scholar] [CrossRef]
- Chai, X.P.; Sun, G.L.; Fang, Y.F.; Hu, L.H.; Liu, X.; Zhang, X.W. Tumor-targeting efficacy of a BF211 prodrug through hydrolysis by fibroblast activation protein-alpha. Acta Pharmacol. Sin. 2018, 39, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Zuo, W.; Li, N.; Zhao, Y.; Fu, T.; Fei, W.; Yu, R.; Yang, J. Synchronized release of bufadienolides in a stable Lutrol F127 based solid dispersion prepared with spray congealing. Drug Dev. Ind. Pharm. 2018, 44, 1817–1825. [Google Scholar] [CrossRef]
- Guo, T.; Song, H.T.; Zhao, M.H.; Zhang, R.H.; Li, X. Preparation of β-cyclodextrin inclusion complex of toad venom. China J. Chin. Mater. Med. 2002, 11, 34–36. [Google Scholar]
- Zou, A.; Zhao, X.; Handge, U.A.; Garamus, V.M.; Willumeit-Romer, R.; Yin, P. Folate receptor targeted bufalin/beta-cyclodextrin supramolecular inclusion complex for enhanced solubility and anti-tumor efficiency of bufalin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 609–618. [Google Scholar] [CrossRef]
- Zhu, Y.; Wen, L.M.; Li, R.; Dong, W.; Jia, S.Y.; Qi, M.C. Recent advances of nano-drug delivery system in oral squamous cell carcinoma treatment. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9445–9453. [Google Scholar] [PubMed]
- Kumar, S.; Dilbaghi, N.; Saharan, R.; Bhanjana, G. Nanotechnology as Emerging Tool for Enhancing Solubility of Poorly Water-Soluble Drugs. BioNanoScience 2012, 2, 227–250. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Nanoparticles Formulations of Artemisinin and Derivatives as Potential Therapeutics for the Treatment of Cancer, Leishmaniasis and Malaria. Pharmaceutics 2020, 12, 748. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A Successful Tool to Increase Solubility, Stability and Optimise Bioefficacy of Natural Constituents. Curr. Med. Chem. 2019, 26, 4631–4656. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, L.; Ye, S.; Kang, Y.; Liu, J.; Zeng, S.; Yu, L. Research and development of drug delivery systems based on drug transporter and nano-formulation. Asian J. Pharm. Sci. 2020, 15, 220–236. [Google Scholar] [CrossRef]
- Yu, H.P.; Aljuffalic, I.A.; Fang, J.Y. Injectable Drug-Loaded Nanocarriers for Lung Cancer Treatments. Curr. Pharm. Des. 2017, 23, 481–494. [Google Scholar] [CrossRef]
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-Guided Delivery of Nanoparticles to Improve Drug Permeation across Cellular Barriers and Drug Exposure to Selective Cell Types. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wu, Y.; Hu, Y.; Li, X.; Zhao, M.; Lv, Z. Targeted Nanoparticle Drug Delivery System for the Enhancement of Cancer Immunotherapy. J. Biomed. Nanotechnol. 2019, 15, 1839–1866. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gorain, B.; Low, S.Y.; Tan, S.A.; Ling, E.C.S.; Lim, Y.K.; Chin, C.M.; Lee, P.Y.; Lee, C.M.; Ooi, C.H.; et al. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res. Clin. Pr. 2018, 136, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Gorain, B.; Choudhury, H.; Pandey, M.; Kesharwani, P. Paclitaxel loaded vitamin E-TPGS nanoparticles for cancer therapy. Mater. Sci. Eng. C 2018, 91, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sharma, G.; Ghoshal, G.; Kesharwani, P.; Singh, B.; Shivhare, U.S.; Katare, O.P. Lycopene loaded whey protein isolate nanoparticles: An innovative endeavor for enhanced bioavailability of lycopene and anti-cancer activity. Int. J. Pharm. 2018, 546, 97–105. [Google Scholar] [CrossRef]
- Varma, L.T.; Singh, N.; Gorain, B.; Choudhury, H.; Tambuwala, M.M.; Kesharwani, P.; Shukla, R. Recent Advances in Self-Assembled Nanoparticles for Drug Delivery. Curr. Drug Deliv. 2020, 17, 279–291. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Y.; Yuan, Z.; Bao, Y.; Li, R.; Liu, C.; Qiu, Y.; Xu, K.; Shi, X.; Yu, H.; et al. Bufalin-Loaded CaP/DPPE-PEG-EGF Nanospheres: Preparation, Cellular Uptake, Distribution, and Anti-Tumor Effects. J. Biomed. Nanotechnol. 2019, 15, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liang, B.; Sun, Y.; Guo, X.L.; Bao, Y.J.; Xie, D.H.; Zhou, M.; Duan, Y.R.; Yin, P.H.; Peng, Z.H. Preparation of bufalin-loaded pluronic polyetherimide nanoparticles, cellular uptake, distribution, and effect on colorectal cancer. Int. J. Nanomed. 2014, 9, 4035–4041. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Q.; Huang, N.; Yang, G.L.; Lin, Q.; Su, Y.H. Bufalin-loaded bovine serum albumin nanoparticles demonstrated improved anti-tumor activity against hepatocellular carcinoma: Preparation, characterization, pharmacokinetics and tissue distribution. Oncotarget 2017, 8, 63311–63323. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Yin, Z.F.; Sheng, J.Y.; Jiang, Z.Q.; Wu, B.Y.; Su, Y.H. A comparison study of pharmacokinetics between bufalin-loaded bovine serum albumin nanoparticles and bufalin in rats. J. Chin. Integr. Med. 2012, 10, 674–680. [Google Scholar] [CrossRef]
- Yin, P.; Wang, Y.; Qiu, Y.; Hou, L.; Liu, X.; Qin, J.; Duan, Y.; Liu, P.; Qiu, M.; Li, Q. Bufalin-loaded mPEG-PLGA-PLL-cRGD nanoparticles: Preparation, cellular uptake, tissue distribution, and anticancer activity. Int. J. Nanomed. 2012, 7, 3961–3969. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, L.; Chen, P.; Chen, M.; Zheng, M.; Shi, F.; Wang, Y. Tumor-Targeted Delivery of Bufalin-Loaded Modified Albumin-Polymer Hybrid for Enhanced Antitumor Therapy and Attenuated Hemolysis Toxicity and Cardiotoxicity. AAPS PharmSciTech 2021, 22, 137. [Google Scholar] [CrossRef]
- Fan, J.; Liu, B.; Long, Y.; Wang, Z.; Tong, C.; Wang, W.; You, P.; Liu, X. Sequentially-targeted biomimetic nano drug system for triple-negative breast cancer ablation and lung metastasis inhibition. Acta Biomater. 2020, 113, 554–569. [Google Scholar] [CrossRef]
- Dong, H.; Tian, L.; Gao, M.; Xu, H.; Zhang, C.; Lv, L.; Zhang, J.; Wang, C.; Tian, Y.; Ma, X. Promising galactose-decorated biodegradable poloxamer 188-PLGA diblock copolymer nanoparticles of resibufogenin for enhancing liver cancer therapy. Drug Deliv. 2017, 24, 1302–1316. [Google Scholar] [CrossRef] [Green Version]
- Chu, Q.; Xu, H.; Gao, M.; Guan, X.; Liu, H.; Deng, S.; Huo, X.; Liu, K.; Tian, Y.; Ma, X. Liver-targeting Resibufogenin-loaded poly(lactic-co-glycolic acid)-D-alpha-tocopheryl polyethylene glycol 1000 succinate nanoparticles for liver cancer therapy. Int. J. Nanomed. 2016, 11, 449–463. [Google Scholar]
- Li, J.; Zhang, Z.; Deng, H.; Zheng, Z. Cinobufagin-Loaded and Folic Acid-Modified Polydopamine Nanomedicine Combined with Photothermal Therapy for the Treatment of Lung Cancer. Front. Chem. 2021, 9, 637754. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, W.; Fan, J.; Long, Y.; Xiao, F.; Daniyal, M.; Tong, C.; Xie, Q.; Jian, Y.; Li, B.; et al. RBC membrane camouflaged prussian blue nanoparticles for gamabutolin loading and combined chemo/photothermal therapy of breast cancer. Biomaterials 2019, 217, 119301. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Tong, C.; Fan, J.; Wang, Z.; Xie, Q.; Long, Y.; You, P.; Wang, W.; Liu, B. Biomimetic nanoparticles loading with gamabutolin-indomethacin for chemo/photothermal therapy of cervical cancer and anti-inflammation. J. Control Release 2021, 339, 259–273. [Google Scholar] [CrossRef]
- Ordóñez-Gutiérrez, L.; Wandosell, F. Nanoliposomes as a Therapeutic Tool for Alzheimer’s Disease. Front. Synaptic Neurosci. 2020, 12, 20. [Google Scholar] [CrossRef]
- Mozafari, M.R. Nanoliposomes: Preparation and analysis. Methods Mol. Biol. 2010, 605, 29–50. [Google Scholar] [PubMed]
- Zamani, P.; Momtazi-Borojeni, A.A.; Nik, M.E.; Oskuee, R.K.; Sahebkar, A. Nanoliposomes as the adjuvant delivery systems in cancer immunotherapy. J. Cell. Physiol. 2018, 233, 5189–5199. [Google Scholar] [CrossRef]
- Yoon, G.; Park, J.W.; Yoon, I.-S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): Recent advances in drug delivery. J. Pharm. Investig. 2013, 43, 353–362. [Google Scholar] [CrossRef]
- Gubernator, J. Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, R.; Weng, Y.; Tang, X. Preparation and evaluation of lyophilized liposome-encapsulated bufadienolides. Drug Dev. Ind. Pharm. 2009, 35, 1048–1058. [Google Scholar] [CrossRef]
- Li, F.; Weng, Y.; Wang, L.; He, H.; Yang, J.; Tang, X. The efficacy and safety of bufadienolides-loaded nanostructured lipid carriers. Int. J. Pharm. 2010, 393, 204–212. [Google Scholar] [CrossRef]
- Hu, K.; Zhu, L.; Liang, H.; Hu, F.; Feng, J. Improved antitumor efficacy and reduced toxicity of liposomes containing bufadienolides. Arch. Pharmacal Res. 2011, 34, 1487–1494. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, L.; He, F.; Chen, J.; Zhao, M.; Li, S.; Wu, H.; Liu, Y.; Zhang, Y.; Ping, Q.; et al. Surfactant Assisted Rapid-Release Liposomal Strategies Enhance the Antitumor Efficiency of Bufalin Derivative and Reduce Cardiotoxicity. Int. J. Nanomed. 2021, 16, 3581–3598. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhou, X.; Cao, W.; Bi, L.; Zhang, Y.; Yang, Q.; Wang, S. Improved Antitumor Efficacy and Pharmacokinetics of Bufalin via PEGylated Liposomes. Nanoscale Res. Lett. 2017, 12, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Liu, J. Transferrin and folic acid co-modified bufalin-loaded nanoliposomes: Preparation, characterization, and application in anti-cancer activity. Int. J. Nanomed. 2018, 13, 6009–6018. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, P.; Sun, C.; Feng, N.; Zhou, W.; Yang, Y.; Tan, R.; Chen, Z.; Wu, S.; Zhao, J. Wheat germ agglutinin-grafted lipid nanoparticles: Preparation and in vitro evaluation of the association with Caco-2 monolayers. Int. J. Pharm. 2010, 397, 155–163. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Sun, C.; Zhao, J.; Du, Y.; Shi, F.; Feng, N. Bioadhesion and enhanced bioavailability by wheat germ agglutinin-grafted lipid nanoparticles for oral delivery of poorly water-soluble drug bufalin. Int. J. Pharm. 2011, 419, 260–265. [Google Scholar] [CrossRef]
- Yuan, Z.; Yuan, Y.; Han, L.; Qiu, Y.; Huang, X.; Gao, F.; Fan, G.; Zhang, Y.; Tang, X.; He, X.; et al. Bufalin-loaded vitamin E succinate-grafted-chitosan oligosaccharide/RGD conjugated TPGS mixed micelles demonstrated improved antitumor activity against drug-resistant colon cancer. Int. J. Nanomed. 2018, 13, 7533–7548. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Cai, H.; Yin, T.; Huo, M.; Ma, P.; Zhou, J.; Lai, W. Paclitaxel-loaded redox-sensitive nanoparticles based on hyaluronic acid-vitamin E succinate conjugates for improved lung cancer treatment. Int. J. Nanomed. 2018, 13, 1585–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Xie, Q.; Su, K.; Li, Z.; Dong, D.; Wu, B. Systemic delivery of the anticancer agent arenobufagin using polymeric nanomicelles. Int. J. Nanomed. 2017, 12, 4981–4989. [Google Scholar] [CrossRef] [Green Version]
- Gou, H.; Huang, R.-C.; Zhang, F.-L.; Su, Y.-H. Design of dual targeting immunomicelles loaded with bufalin and study of their anti-tumor effect on liver cancer. J. Integr. Med. 2021, 19, 408–417. [Google Scholar] [CrossRef]
- Ramos-Bell, S.; Calderón-Santoyo, M.; Barros-Castillo, J.C.; Ragazzo-Sánchez, J.A. Characterization of submicron emulsion processed by ultrasound homogenization to protect a bioactive extract from sea grape (Coccoloba uvifera L.). Food Sci. Biotechnol. 2020, 29, 1365–1372. [Google Scholar] [CrossRef]
- Li, W.; Lin, X.; Yang, Z.; Zhang, W.; Ren, T.; Qu, F.; Wang, Y.; Zhang, N.; Tang, X. A bufadienolide-loaded submicron emulsion for oral administration: Stability, antitumor efficacy and toxicity. Int. J. Pharm. 2015, 479, 52–62. [Google Scholar] [CrossRef]
- Tartaro, G.; Mateos, H.; Schirone, D.; Angelico, R.; Palazzo, G. Microemulsion Microstructure(s): A Tutorial Review. Nanomaterials 2020, 10, 1657. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.Q.; Zhang, X.; Feng, N.P.; Zhao, J.H.; Wu, S.; Tan, R. An Improved Formulation Screening and Optimization Method Applied to the Development of a Self-Microemulsifying Drug Delivery System. Chem. Pharm. Bull. 2010, 58, 6–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Angelova, A.; Liu, J.; Garamus, V.M.; Li, N.; Drechsler, M.; Gong, Y.; Zou, A. In situ phase transition of microemulsions for parenteral injection yielding lyotropic liquid crystalline carriers of the antitumor drug bufalin. Colloids Surf. B Biointerfaces 2019, 173, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, J.Y.C.; Wang, H. Introduction for Design of Nanoparticle Based Drug Delivery Systems. Curr. Pharm. Des. 2017, 23, 2108–2112. [Google Scholar] [CrossRef]
- Tekade, R.K.; Dutta, T.; Gajbhiye, V.; Jain, N.K. Exploring dendrimer towards dual drug delivery: pH responsive simultaneous drug-release kinetics. J. Microencapsul. 2009, 26, 287–296. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Akhtar, S.; Al-Zaid, B.; El-Hashim, A.Z.; Chandrasekhar, B.; Attur, S.; Benter, I.F. Impact of PAMAM delivery systems on signal transduction pathways in vivo: Modulation of ERK1/2 and p38 MAP kinase signaling in the normal and diabetic kidney. Int. J. Pharm. 2016, 514, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-o.; Jing, J.; Xiao, W.; Tan, Z.; Lv, Q.; Yang, J.; Chen, S. Enhanced Intestinal Permeability of Bufalin by a Novel Bufalin-Peptide-Dendrimer Inclusion through Caco-2 Cell Monolayer. Molecules 2017, 22, 2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, D.; Khurana, B.; Rath, G.; Nanda, S.; Goyal, A.K. Recent Advances in Nanosuspension Technology for Drug Delivery. Curr. Pharm. Des. 2018, 24, 2403–2415. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar]
- Zuo, W.; Qu, W.; Li, N.; Yu, R.; Hou, Y.; Liu, Y.; Gou, G.; Yang, J. Fabrication of multicomponent amorphous bufadienolides nanosuspension with wet milling improves dissolution and stability. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1513–1522. [Google Scholar] [CrossRef] [Green Version]
- Varuna Kumara, J.B.; Ravikumara, N.R.; Madhusudhan, B. Evaluation of Surfactants-Assisted Folic Acid-Loaded Pectin Submicrospheres: Characterization and Hemocompatibility Assay. Indian J. Clin. Biochem. 2016, 31, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Lai, X.; Li, L.; Hu, Z.; Wang, S.; Halpert, J.E.; Yu, R.; Wang, D. Water-soluble monodispersed lanthanide oxide submicrospheres: PVP-assisted hydrothermal synthesis, size-control and luminescence properties. Chemphyschem 2012, 13, 2610–2614. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Liu, Q.; Lu, S.; Chen, X.; Xu, W.; Shi, F. Co-delivery of bufalin and nintedanib via albumin sub-microspheres for synergistic cancer therapy. J. Control Release 2021, 338, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Sun, S.; Li, X.; Lv, S.; Xu, T.; Sun, J.; Feng, W.; Zhang, L.; Li, Y. Preparation, in vitro and in vivo evaluation of mPEG-PLGA nanoparticles co-loaded with syringopicroside and hydroxytyrosol. J. Mater. Sci. Mater. Med. 2016, 27, 24. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Guan, Q.X.; Shang, E.Y.; Xiao, H.B.; Yu, X.; Shi, L.; Zhao, C.C.; Guo, Y.Y.; Lv, S.W.; Li, Y.J. Hyaluronic acid-coated nanostructured lipid carriers for loading multiple traditional Chinese medicine components for liver cancer treatment. Pak. J. Pharm. Sci. 2020, 33, 109–119. [Google Scholar] [PubMed]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, H.; Li, B.; Li, H.; Gao, L.; Zhang, C.; Sheng, H.; Zhu, L. Novel Strategies for Solubility and Bioavailability Enhancement of Bufadienolides. Molecules 2022, 27, 51. https://doi.org/10.3390/molecules27010051
Shao H, Li B, Li H, Gao L, Zhang C, Sheng H, Zhu L. Novel Strategies for Solubility and Bioavailability Enhancement of Bufadienolides. Molecules. 2022; 27(1):51. https://doi.org/10.3390/molecules27010051
Chicago/Turabian StyleShao, Huili, Bingqian Li, Huan Li, Lei Gao, Chao Zhang, Huagang Sheng, and Liqiao Zhu. 2022. "Novel Strategies for Solubility and Bioavailability Enhancement of Bufadienolides" Molecules 27, no. 1: 51. https://doi.org/10.3390/molecules27010051