Comparison of Volatile Organic Compounds of Sideritis romana L. and Sideritis montana L. from Croatia
Abstract
:1. Introduction
2. Results
2.1. HS-SPME/GC-MS Analysis
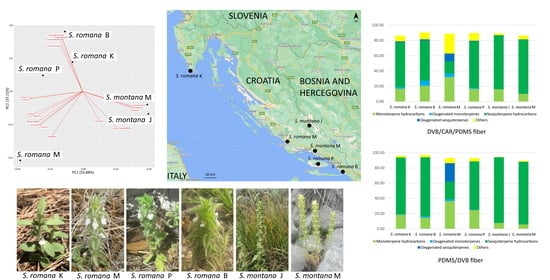
2.1.1. DVB/CAR/PDMS Fiber
2.1.2. PDMS/DVB Fiber
2.2. Principal Component Analysis
2.2.1. DVB/CAR/PDMS Fiber
2.2.2. PDMS/DVB Fiber
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. SPME Fibers and Extraction Procedure
4.3. GC-MS Analysis
4.4. Principal Component Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Sadgrove, N.; Jones, G. A contemporary introduction to essential oils: Chemistry, bioactivity and prospects for Australian agriculture. Agriculture 2015, 5, 48–102. [Google Scholar] [CrossRef] [Green Version]
- de Mesquita, L.S.S.; Luz, T.R.S.A.; de Mesquita, J.W.C.; Coutinho, D.F.; do Amaral, F.M.M.; de Sousa Ribeiro, M.N.; Malik, S. Exploring the anticancer properties of essential oils from family Lamiaceae. Food Rev. Int. 2019, 35, 105–131. [Google Scholar] [CrossRef]
- Paibon, W.; Yimnoi, C.-A.; Tembab, N.; Boonlue, W.; Jampachaisri, K.; Nuengchamnong, N.; Waranuch, N.; Ingkaninan, K. Comparison and evaluation of volatile oils from three different extraction methods for some Thai fragrant flowers. Int. J. Cosmet. Sci. 2011, 33, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Sagratini, G.; Maggi, F.; Bílek, T.; Papa, F.; Vittori, S. Analysis of the volatile compounds of Teucrium flavum L. subsp. flavum (Lamiaceae) by headspace solid-phase microextraction coupled to gas chromatography with flame ionisation and mass spectrometric detection. Nat. Prod. Res. 2012, 26, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.-U.; Latief, R.; Bhat, K.A.; Khuroo, M.A.; Shawl, A.S.; Chandra, S. Comparative analysis of the aroma chemicals of Melissa officinalis using hydrodistillation and HS-SPME techniques. Arab. J. Chem. 2017, 10, S2485–S2490. [Google Scholar] [CrossRef] [Green Version]
- El-Sakhawy, F.S.; Kassem, H.A.; El-Gayed, S.H.; Mostafa, M.M. Headspace solid phase microextraction analysis of volatile compounds of the aerial parts and flowers of Plectranthus neochilus Schltr. and Salvia farinacea Benth. J. Essent. Oil Bear. Plants 2018, 21, 674–686. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Radonić, A.; Zekić, M.; Kranjac, M. The application of headspace solid-phase microextraction as a preparation approach for gas chromatography with mass spectrometry. Kem. Ind. 2020, 69, 515–520. [Google Scholar] [CrossRef]
- Fraga, B.M. Phytochemistry and chemotaxonomy of Sideritis species from the Mediterranean region. Phytochemistry 2012, 76, 7–24. [Google Scholar] [CrossRef]
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef]
- Sideritis L. Available online: http://www.worldfloraonline.org/taxon/wfo-4000035314 (accessed on 15 July 2021).
- Aneva, I.; Zhelev, P.; Kozuharova, E.; Danova, K.; Nabavi, S.F.; Behzad, S. Genus Sideritis, section Empedoclia in southeastern Europe and Turkey—studies in ethnopharmacology and recent progress of biological activities. DARU J. Pharm. Sci. 2019, 27, 407–421. [Google Scholar] [CrossRef]
- Cavalcanti, M.R.M.; Passos, F.R.S.; Monteiro, B.S.; Gandhi, S.R.; Heimfarth, L.; Lima, B.S.; Nascimento, Y.M.; Duarte, M.C.; Araujo, A.A.S.; Menezes, I.R.A.; et al. HPLC-DAD-UV analysis, anti-inflammatory and anti-neuropathic effects of methanolic extract of Sideritis bilgeriana (lamiaceae) by NF-κB, TNF-α, IL-1β and IL-6 involvement. J. Ethnopharmacol. 2021, 265, 113338. [Google Scholar] [CrossRef] [PubMed]
- Deveci, E.; Tel-Çayan, G.; Duru, M.E.; Öztürk, M. Phytochemical contents, antioxidant effects, and inhibitory activities of key enzymes associated with Alzheimer’s disease, ulcer, and skin disorders of Sideritis albiflora and Sideritis leptoclada. J. Food Biochem. 2019, 43, e13078. [Google Scholar] [CrossRef] [PubMed]
- Bojović, D.; Janković, S.; Potpara, Z.; Tadić, V. Summary of the phytochemical research performed to date on Sideritis species. Ser. J. Exp. Clin. Res. 2011, 12, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Solomou, A.D.; Skoufogianni, E.; Mylonas, C.; Germani, R.; Danalatos, N.G. Cultivation and utilization of “Greek mountain tea” (Sideritis spp.): Current knowledge and future challenges. Asian J. Agric. Biol. 2019, 7, 289–299. [Google Scholar]
- Domac, R. Flora Hrvatske, 2nd ed.; Školska Knjga: Zagreb, Croatia, 2002; pp. 291–292. [Google Scholar]
- Kirimer, N.; Tabanca, N.; Özek, T.; Tümen, G.; Başer, K.H.C. Essential oils of annual Sideritis species growing in Turkey. Pharm. Biol. 2000, 38, 106–111. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I. Characterization of the volatile fraction of a Sideritis romana population of Montemarcello (Eastern Liguria). J. Essent. Oil Res. 1994, 6, 239–242. [Google Scholar] [CrossRef]
- Tadić, V.; Oliva, A.; Božović, M.; Cipolla, A.; De Angelis, M.; Vullo, V.; Garzoli, S.; Ragno, R. Chemical and antimicrobial analyses of Sideritis romana L. subsp. purpurea (Tal. ex Benth.) Heywood, an endemic of the Western Balkan. Molecules 2017, 22, 1395. [Google Scholar] [CrossRef] [Green Version]
- Koutsaviti, A.; Bazos, I.; Milenković, M.; Pavlović-Drobac, M.; Tzakou, O. Antimicrobial activity and essential oil composition of five Sideritis taxa of Empedoclia and Hesiodia sect. from Greece. Rec. Nat. Prod. 2013, 7, 6–14. [Google Scholar]
- Todorova, M.N.; Christov, R.C. Essential oil composition of three Sideritis species from Bulgaria. J. Essent. Oil Res. 2000, 12, 418–420. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Frezza, C.; Serafini, M.; Giacomello, G.; Giuliani, C.; Bramucci, M.; Quassinti, L.; Lupidi, G.; Lucarini, D.; et al. Secondary metabolites, glandular trichomes and biological activity of Sideritis montana L. subsp. montana from Central Italy. Chem. Biodivers. 2016, 13, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Kilic, O. Essential oil composition of two Sideritis L. taxa from Turkey: A chemotaxonomic approach. Asian J. Chem. 2014, 26, 2466–2470. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilić, B.S.; Mihajilov-Krstev, T.M.; Nikolić, N.D.; Milosavljević, V.N.; Nikolić, D.M. Antibakterijski potencijal etarskog ulja Sideritis montana L. (Lamiaceae). Hem. Ind. 2012, 66, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Meshkatalsadat, M.H.; Sarabi, R.S.; Akbari, N.; Pireai, M. Composition of essential oils from Sideritis montana of Iran. Asian J. Chem. 2007, 19, 5769–5771. [Google Scholar]
- Garzoli, S.; Božović, M.; Baldisserotto, A.; Andreotti, E.; Pepi, F.; Tadić, V.; Manfredini, S.; Ragno, R. Sideritis romana L. subsp. purpurea (Tal. ex Benth.) Heywood, a new chemotype from Montenegro. Nat. Prod. Res. 2017, 32, 1056–1061. [Google Scholar] [CrossRef]
- Uysal, T.; Sezer, E.N.Ş.; Bozkurt, M. Headspace solid phase microextraction (HS-SPME) analysis of Sideritis ozturkii Aytac & Aksoy. In Proceedings of the 5th International Mediterranean Symposium on Medicinal and Aromatic Plants Book, Cappadocia, Turkey, 24–26 April 2019. [Google Scholar]
- Qazimi, B.; Stefkov, G.; Karapandzova, M.; Cvetkovikj, I.; Kulevanova, S. Aroma compounds of mountain tea (Sideritis scardica and S. raeseri) from Western Balkan. Nat. Prod. Commun. 2014, 9, 1369–1372. [Google Scholar] [CrossRef] [Green Version]
- Topçu, G.; Barla, A.; Gören, A.C.; Bilsel, G.; Bilsel, M.; Tümen, G. Analysis of the essential oil composition of Sideritis albiflora using direct thermal desorption and headspace GC-MS techniques. Turk. J. Chem. 2005, 29, 525–529. [Google Scholar]
- Aboukhalid, K.; Al Faiz, C.; Douaik, A.; Bakha, M.; Kursa, K.; Agacka-Mołdoch, M.; Machon, N.; Tomi, F.; Lamiri, A. Influence of environmental factors on essential oil variability in Origanum compactum Benth. growing wild in Morocco. Chem. Biodivers. 2017, 14, e1700158. [Google Scholar] [CrossRef]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef]
- Wajs, A.; Pranovich, A.; Reunanen, M.; Willför, S.; Holmbom, B. Characterisation of volatile organic compounds in stemwood using solid-phase microextraction. Phytochem. Anal. 2006, 17, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Dadalı, C.; Elmacı, Y. Optimization of headspace-solid phase microextraction (HS-SPME) technique for the analysis of volatile compounds of margarine. J. Food Sci. Technol. 2019, 56, 4834–4843. [Google Scholar] [CrossRef]
- Pinheiro, G.P.; Galbiatti, M.I.; Carneiro, M.J.; Sawaya, A.C.H.F. Comparison of four different solid-phase microextraction fibers for analysis of Plectranthus amboinicus (Lour.) Spreng. leaf volatiles. Adv. Med. Plant. Res. 2019, 7, 38–43. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z. A short review of headspace extraction and ultrasonic solvent extraction for honey volatiles fingerprinting. Croat. J. Food. Sci. Technol. 2009, 1, 28–34. [Google Scholar]
- Wang, H.-Y.; Zhang, W.; Dong, J.-H.; Wu, H.; Wang, Y.-H.; Xiao, H.-X. Optimization of SPME–GC–MS and characterization of floral scents from Aquilegia japonica and A. amurensis flowers. BMC Chem. 2021, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Sukkaew, S.; Pripdeevech, P.; Thongpoon, C.; Machan, T.; Wongchuphan, R. Volatile constituents of Murraya koenigii fresh leaves using headspace solid phase microextraction—gas chromatography—mass spectrometry. Nat. Prod. Commun. 2014, 9, 1783–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyota, M.; Koyama, H.; Mizutani, M.; Asakawa, Y. (−)-ent-spathulenol isolated from liverworths is an artefact. Phytochemistry 1996, 41, 1347–1350. [Google Scholar] [CrossRef]
- Tadić, V.; Bojović, D.; Arsić, I.; Đorđević, S.; Aksentijevic, K.; Stamenić, M.; Janković, S. Chemical and antimicrobial evaluation of supercritical and conventional Sideritis scardica Griseb., Lamiaceae extracts. Molecules 2012, 17, 2683–2703. [Google Scholar] [CrossRef] [Green Version]
- Jakab, E.; Sebestyén, Z.; Babinszki, B.; Barta-Rajnai, E.; Czégény, Z.; Nicol, J.; Clayton, P.; Liu, C. Thermo-oxidative decomposition of lovage (Levisticum officinale) and davana (Artemisia pallens) essential oils under simulated tobacco heating product conditions. Beitr. Tabakforsch. Int. 2020, 29, 27–43. [Google Scholar] [CrossRef]
- Tran, D.N.; Cramer, N. Biomimetic synthesis of (+)-ledene, (+)-viridiflorol, (−)-palustrol, (+)-spathulenol, and psiguadial A, C, and D via the platform terpene (+)-bicyclogermacrene. Chem. Eur. J. 2014, 20, 10654–10660. [Google Scholar] [CrossRef]
- Carvalho, F.A.; Oda, F.B.; Crevelin, E.J.; Crotti, A.E.M.; dos Santos, A.G. Casearia sylvestris essential oil degradation products generated by leaf processing. Chem. Biodivers. 2021, 18, e2000880. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Ukeda, H.; Sawamura, M. Changes in the volatile composition of yuzu (Citrus junos Tanaka) cold-pressed oil during storage. J. Agric. Food Chem. 1996, 44, 550–556. [Google Scholar] [CrossRef]
- Šegota, T.; Filipčić, A. Köppenova podjela klima i hrvatsko nazivlje. Geoadria 2003, 8, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Kirimer, N.; Baser, K.H.C.; Demirci, B.; Duman, H. Essential oils of Sideritis species of Turkey belonging to the section Empedoclia. Chem. Nat. Comp. 2004, 40, 19–23. [Google Scholar] [CrossRef]
- Kirimer, N.; Demirci, B.; Iscan, G.; Baser, K.H.C.; Duman, H. Composition of the essential oils of two Sideritis species from Turkey and antimicrobial activity. Chem. Nat. Comp. 2008, 44, 121–123. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in terpene profiles of Thymus vulgaris in water deficit stress response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef] [Green Version]
- Kigathi, R.N.; Weisser, W.W.; Reichelt, M.; Gershenzon, J.; Unsicker, S.B. Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol. 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Valerio, F.; Gannouchi, A.; Trani, A.; Mezzapesa, G. Ecological and plant community implication on essential oils composition in useful wild officinal species: A pilot case study in Apulia (Italy). Plants 2021, 10, 574. [Google Scholar] [CrossRef]
- Topić, J.; Šegulja, N. Floristic and ecological characteristics of the southernmost part of Istria (Croatia). Acta Bot. Croat. 2000, 59, 179–200. [Google Scholar]
- Bogdanović, S.; Iveša, N.; Temunović, M.; Ljubičić, I. Plant diversity of Gornji Kamenjak (Istria, Croatia). Agric. Conspec. Sci. 2018, 83, 195–204. [Google Scholar]
- Milović, M.; Pandža, M.; Jasprica, N.; Tafra, D.; Krpina, V. The vascular flora of Mt Svilaja (Outer Dinarides, South Croatia). Nat. Croat. 2021, 30, 85–144. [Google Scholar] [CrossRef]
- Milović, M. The flora of Šibenik and its surroundings. Nat. Croat. 2002, 11, 171–223. [Google Scholar]
- Tafra, D.; Pandža, M.; Milović, M. Vacular flora of the town of Omiš. Nat. Croat. 2012, 21, 301–334. [Google Scholar]
- Vladović, D.; Ilijanić, L.J. Treći prilog flori planine Mosor (Hrvatska). Acta Bot. Croat. 1995, 54, 41–46. [Google Scholar]
- Štamol, V.; Marković, L.J. Prilog flori otoka Brača. Acta Bot. Croat. 1985, 44, 99–106. [Google Scholar]
- Trinajstić, I. Vaskularna flora otoka Hvara. Acta Bot. Croat. 1993, 52, 113–143. [Google Scholar]
- Bedalov, M. Prilog flori otoka Šolte. Acta Bot. Croat. 1989, 48, 121–127. [Google Scholar]
- Moradi, M.; Kaykhaii, M.; Ghiasvand, A.R.; Shadabi, S.; Salehinia, A. Comparison of headspace solid-phase microextraction, headspace single-drop microextraction and hydrodistillation for chemical screening of volatiles in Myrtus communis L. Phytochem. Anal. 2012, 23, 379–386. [Google Scholar] [CrossRef]
- Skalicka-Wozniak, K.; Los, R.; Glowniak, K.; Malm, A. Comparison of hydrodistillation and headspace solid-phase microextraction techniques for antibacterial volatile compounds from the fruits of Seseli libanotis. Nat. Prod. Commun. 2010, 5, 1427–1430. [Google Scholar] [CrossRef] [Green Version]
- Farouk, A.; Ali, H.; Al-Khalifa, A.R.; Mohsen, M.; Fikry, R. Aroma volatile compounds of parsley cultivated in the Kingdom of Saudi Arabia and Egypt extracted by hydrodistillation and headspace solid-phase microextraction. Int. J. Food Prop. 2017, 20, S2868–S2877. [Google Scholar] [CrossRef]
- Mékaoui, R.; Benkaci-Ali, F.; Alsafra, Z.; Eppe, G. Volatiles profile of the different parts of Algerian Bupleurum plantagineum Desf. by headspace solid-phase microextraction and hydrodistillation. Nat. Prod. Res. 2020, 34, 3134–3138. [Google Scholar] [CrossRef]
- Grecco, S.S.; Martins, E.G.A.; Girola, N.; de Figueiredo, C.R.; Matsuo, A.L.; Soares, M.G.; Bertoldo, B.C.; Sartorelli, P.; Lago, J.H.G. Chemical composition and in vitro cytotoxic effects of the essential oil from Nectandra leucantha leaves. Pharm. Biol. 2015, 53, 133–137. [Google Scholar] [CrossRef]
- da Silva, E.B.P.; Matsuo, A.L.; Figueiredo, C.R.; Chaves, M.H.; Sartorelli, P.; Lago, J.H.G. Chemical constituents and cytotoxic evaluation of essential oils from leaves of Porcelia macrocarpa (Annonaceae). Nat. Prod. Commun. 2013, 8, 277–279. [Google Scholar] [CrossRef] [Green Version]
- Noriega, P.; Guerrini, A.; Sacchetti, G.; Grandini, A.; Ankuash, E.; Manfredini, S. Chemical composition and biological activity of five essential oils from the Ecuadorian Amazon rain forest. Molecules 2019, 24, 1637. [Google Scholar] [CrossRef] [Green Version]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Mashayekhi, H.A.; Rezaee, M.; Garmaroudi, S.S.; Montazeri, N.; Ahmadi, S.J. Rapid and sensitive determination of benzaldehyde arising from benzyl alcohol used as preservative in an injectable formulation solution using dispersive liquid–liquid microextraction followed by gas chromatography. Anal. Sci. 2011, 27, 865–868. [Google Scholar] [CrossRef] [Green Version]
- Almoughrabie, S.; Ngari, C.; Guillier, L.; Briandet, R.; Poulet, V.; Dubois-Brissonnet, F. Rapid assessment and prediction of the efficiency of two preservatives against S. aureus in cosmetic products using High Content Screening—Confocal Laser Scanning Microscopy. PLoS ONE 2020, 15, e0236059. [Google Scholar] [CrossRef]
- Trevizan, L.N.T.; do Nascimento, K.F.; Santos, J.A.; Kassuya, C.A.L.; Cardoso, C.A.L.; Vieira, M.C.; Moreira, F.M.F.; Croda, J.; Formagio, A.S.N. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. J. Ethnopharmacol. 2016, 192, 510–515. [Google Scholar] [PubMed]
- Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical composition and immunomodulatory activity of essential oils from Rhododendron albiflorum. Molecules 2021, 26, 3652. [Google Scholar] [CrossRef] [PubMed]
- Trikka, F.; Michailidou, S.; Makris, A.M.; Argiriou, A. Biochemical fingerprint of Greek Sideritis spp.: Implications for potential drug discovery and advanced breeding strategies. Med. Aromat. Plants 2019, 8, 335. [Google Scholar] [CrossRef] [Green Version]
- Koedam, A. Volatile oil composition of Greek mountain tea (Sideritis spp.). J. Sci. Food Agric. 1986, 36, 681–684. [Google Scholar] [CrossRef]
- Pljevljakušić, D.; Šavikin, K.; Janković, T.; Zdunić, G.; Ristić, M.; Godjevac, D.; Konić-Ristić, A. Chemical properties of the cultivated Sideritis raeseri Boiss. & Heldr. subsp. raeseri. Food Chem. 2011, 124, 226–233. [Google Scholar]
- Assessment report on Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-sideritis-scardica-griseb-sideritis-clandestina-bory-chaub-hayek-sideritis_en.pdf (accessed on 24 August 2021).
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Roje, M.; Jokić, S. Chemical diversity of headspace and volatile oil composition of two brown algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules 2019, 24, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerković, I.; Cikoš, A.-M.; Babić, S.; Čižmek, L.; Bojanić, K.; Aladić, K.; Ul’yanovskii, N.V.; Kosyakov, D.S.; Lebedev, A.T.; Čož-Rakovac, R.; et al. Bioprospecting of less-polar constituents from endemic brown macroalga Fucus virsoides J. Agardh from the Adriatic Sea and targeted antioxidant effects in vitro and in vivo (zebrafish model). Mar. Drugs 2021, 19, 235. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2021; Available online: https://www.rstudio.com/ (accessed on 24 August 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 24 August 2021).
- Tang, Y.; Horikoshi, M.; Li, W. ggfortify: Unified Interface to Visualize Statistical Results of Popular R Packages. R J. 2016, 8, 474–485. [Google Scholar] [CrossRef] [Green Version]
- Horikoshi, M.; Tang, Y. ggfortify: Data Visualization Tools for Statistical Analysis Results. 2016. Available online: https://CRAN.R-project.org/package=ggfortify (accessed on 24 August 2021).





| No. | Compound | CD 1 | RI 2 | S. romana K | S. romana B | S. romana M | S. romana P | S. montana J | S. montana M |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Acetic acid | C1 | <900 | - | 0.85 | 3.11 | 1.33 | 0.32 | 0.55 |
| 2 | Pentanal | <900 | 0.15 | 0.40 | - | - | - | - | |
| 3 | Hexanal | <900 | 0.07 | 0.08 | 0.52 | 0.24 | 0.02 | 0.04 | |
| 4 | (E)-Hex-2-enal | <900 | 0.24 | 0.13 | 0.90 | 0.42 | 0.14 | 0.47 | |
| 5 | α-Thujene | C2 | 936 | 0.47 | 1.03 | 1.08 | 0.21 | 0.16 | 0.05 |
| 6 | α-Pinene | C3 | 944 | 1.32 | 0.94 | 2.51 | 2.19 | 3.42 | 1.23 |
| 7 | Camphene | 960 | 0.07 | 0.07 | 0.01 | 0.19 | 0.07 | - | |
| 8 | Benzaldehyde | C4 | 969 | 0.44 | 0.32 | 2.58 | 0.71 | 0.37 | 0.42 |
| 9 | Hexanoic acid | 975 | 0.12 | - | - | - | - | 0.09 | |
| 10 | Sabinene | 981 | 0.27 | 0.44 | 0.21 | 0.09 | 0.13 | 0.09 | |
| 11 | Oct-1-en-3-ol | 983 | 0.60 | - | - | - | 0.13 | 0.46 | |
| 12 | β-Pinene | C5 | 985 | 3.69 | 2.61 | 6.67 | 8.01 | 2.81 | 1.52 |
| 13 | β-Myrcene | C6 | 995 | 0.80 | 0.84 | 1.27 | 0.88 | 0.91 | 0.64 |
| 14 | (E,E)-Hepta-2,4-dienal | 1001 | - | 0.07 | 0.63 | - | 0.05 | - | |
| 15 | α-Phellandrene | C7 | 1011 | 0.32 | 3.37 | 2.54 | 0.20 | 0.10 | 0.09 |
| 16 | α-Terpinene | C8 | 1023 | 0.62 | 0.61 | 1.32 | 0.38 | 0.07 | 0.07 |
| 17 | p-Cymene | C9 | 1032 | 0.99 | 1.81 | 5.49 | 1.32 | 0.04 | 0.05 |
| 18 | Limonene | C10 | 1036 | 6.34 | 7.58 | 7.10 | 1.68 | 8.14 | 6.31 |
| 19 | Benzyl alcohol | C11 | 1040 | 2.96 | 3.82 | 13.28 | 3.84 | 0.84 | 2.14 |
| 20 | Phenylacetaldehyde | 1051 | 0.12 | - | - | - | - | 0.09 | |
| 21 | γ-Terpinene | C12 | 1066 | 1.75 | 0.98 | 3.67 | 1.40 | 0.08 | 0.08 |
| 22 | Octan-1-ol | C13 | 1075 | 1.37 | 2.22 | 3.55 | 2.50 | 0.35 | 0.29 |
| 23 | α-Terpinolene | 1093 | 0.33 | 0.52 | 0.34 | 0.05 | 0.08 | - | |
| 24 | Linalool | 1103 | - | 0.26 | - | - | - | - | |
| 25 | Nonanal | 1107 | 0.11 | 0.07 | 0.42 | 0.19 | - | 0.09 | |
| 26 | Benzeneethanol | 1117 | 0.17 | 0.13 | - | - | 0.04 | - | |
| 27 | α-Campholenal | 1132 | 0.07 | - | - | - | - | - | |
| 28 | trans-Pinocarveol 3 | 1145 | - | 0.36 | 0.77 | 0.40 | - | - | |
| 29 | Pinocarvone | 1169 | 0.20 | 0.23 | 0.71 | 0.31 | - | - | |
| 30 | 4-Terpineol | 1182 | 0.60 | 0.95 | 0.89 | 0.15 | 0.05 | 0.07 | |
| 31 | Cryptone | 1191 | - | 0.20 | - | - | - | - | |
| 32 | α-Terpineol | C14 | 1195 | 0.50 | 3.85 | 1.25 | 0.02 | 0.04 | - |
| 33 | Myrtenal | C15 | 1199 | 0.39 | 0.54 | 1.03 | 0.47 | - | - |
| 34 | Decanal | 1208 | 0.16 | 0.10 | - | 0.23 | - | - | |
| 35 | Verbenone | 1212 | 0.11 | 0.15 | - | - | - | - | |
| 36 | 2-Phenoxyethanol | 1223 | 0.15 | 0.06 | - | - | - | - | |
| 37 | Carvone | 1249 | 0.12 | - | - | - | - | - | |
| 38 | Bicycloelemene | 1342 | 0.75 | 0.53 | - | 0.40 | 0.22 | 0.41 | |
| 39 | α-Cubebene | 1353 | 0.12 | - | - | - | 0.56 | 0.56 | |
| 40 | α-Copaene | C16 | 1375 | 0.18 | - | - | - | 1.67 | 2.02 |
| 41 | Isoledene | 1377 | 0.37 | 0.29 | - | 0.23 | 0.34 | - | |
| 42 | Longifolene | 1395 | 0.56 | 0.34 | - | 0.28 | 0.37 | - | |
| 43 | α-Gurjunene | C17 | 1412 | 1.17 | 0.95 | 0.61 | 0.85 | 0.12 | 0.41 |
| 44 | Aristolene | C18 | 1422 | 1.08 | 0.91 | - | - | - | - |
| 45 | trans-Caryophyllene | C19 | 1423 | - | - | 3.60 | 23.88 | 11.89 | 6.56 |
| 46 | Calarene | 1436 | 0.33 | 0.31 | - | 0.34 | - | 0.19 | |
| 47 | Aromadendrene | C20 | 1438 | 1.11 | 1.05 | - | 0.96 | 0.23 | 2.87 |
| 48 | Alloaromadendrene | C21 | 1443 | 7.32 | 6.69 | 2.35 | 6.27 | 0.76 | 0.90 |
| 49 | Selina-5,11-diene 3 | C22 | 1447 | 2.52 | 2.28 | - | 1.91 | - | - |
| 50 | γ-Muurolene | 1455 | 0.99 | 0.71 | - | 0.15 | - | - | |
| 51 | trans-β-Farnesene | C23 | 1461 | 5.12 | 12.79 | 1.00 | 5.58 | 4.83 | 3.56 |
| 52 | Isocaryophyllene | C24 | 1471 | 4.70 | 2.39 | - | 3.22 | - | - |
| 53 | γ-Gurjunene | 1476 | 0.93 | 0.62 | - | 0.45 | - | - | |
| 54 | α-Amorphene | C25 | 1480 | 0.58 | - | - | - | 2.84 | 3.31 |
| 55 | Germacrene D | C26 | 1484 | 1.50 | 0.06 | - | - | 23.23 | 17.04 |
| 56 | trans-β-Ionone | 1489 | 0.31 | 0.18 | 0.37 | 0.22 | - | - | |
| 57 | β-Cadinene | C27 | 1494 | 2.19 | 1.68 | - | 1.24 | 0.98 | 1.47 |
| 58 | Ledene | C28 | 1497 | - | - | 8.20 | - | 2.27 | - |
| 59 | Bicyclogermacrene | C29 | 1498 | 23.04 | 20.48 | - | 15.16 | 4.19 | 11.76 |
| 60 | α-Muurolene | C30 | 1502 | 0.61 | 0.18 | - | - | 1.78 | 2.93 |
| 61 | γ-Cadinene | C31 | 1517 | 0.58 | - | - | - | 3.33 | 3.37 |
| 62 | δ-Cadinene | C32 | 1527 | 1.84 | 0.15 | - | - | 7.57 | 8.85 |
| 63 | Dihydroactinidiolide | 1532 | 0.41 | 0.41 | 0.60 | 0.33 | 0.11 | 0.35 | |
| 64 | Cadina-1,4-diene | C33 | 1536 | 0.20 | - | - | - | 0.94 | 1.24 |
| 65 | α-Cadinene | C34 | 1541 | 0.24 | - | - | - | 1.41 | 1.70 |
| 66 | α-Calacorene | C35 | 1547 | 0.47 | 0.04 | - | - | 1.05 | 1.36 |
| 67 | Spathulenol | C36 | 1580 | 0.58 | 1.30 | - | 0.52 | 0.15 | 0.47 |
| 68 | Viridiflorol | C37 | 1595 | 0.42 | 0.36 | 10.29 | 0.20 | 0.08 | 0.21 |
| 69 | α-Cadinol | 1658 | 0.34 | - | - | - | - | - | |
| Total identified [%] | 86.18 | 90.29 | 88.87 | 89.60 | 89.28 | 86.38 | |||
| No. | Compound | CD 1 | RI 2 | S. romana K | S. romana B | S. romana M | S. romana P | S. montana J | S. montana M |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pentanal | <900 | 0.02 | - | - | 0.03 | - | - | |
| 2 | Hexanal | <900 | 0.02 | 0.03 | 0.12 | 0.10 | 0.01 | 0.02 | |
| 3 | (E)-Hex-2-enal | <900 | 0.02 | 0.03 | 0.10 | 0.06 | 0.03 | 0.05 | |
| 4 | α-Thujene | 936 | 0.55 | 0.41 | 0.79 | 0.58 | 0.07 | 0.04 | |
| 5 | α-Pinene | C1′ | 944 | 2.09 | 2.31 | 3.91 | 2.68 | 1.69 | 1.14 |
| 6 | Camphene | 960 | 0.05 | 0.04 | 0.10 | 0.06 | 0.02 | - | |
| 7 | Benzaldehyde | 969 | 0.06 | 0.08 | 0.40 | 0.15 | 0.04 | 0.10 | |
| 8 | Sabinene | 981 | 0.56 | 0.35 | 0.46 | 0.43 | 0.12 | 0.10 | |
| 9 | β-Pinene | C2′ | 985 | 6.78 | 8.01 | 13.26 | 10.35 | 1.80 | 1.46 |
| 10 | β-Myrcene | 995 | 0.55 | 0.40 | 0.99 | 0.80 | 0.28 | 0.20 | |
| 11 | (E,E)-Hepta-2,4-dienal | 1001 | - | 0.05 | 0.12 | 0.07 | 0.02 | 0.03 | |
| 12 | α-Phellandrene | 1011 | 0.15 | 0.83 | 0.27 | 0.22 | 0.02 | - | |
| 13 | α-Terpinene | C3′ | 1023 | 0.53 | - | 1.15 | 1.01 | - | - |
| 14 | p-Cymene | C4′ | 1032 | 0.81 | 0.16 | 3.10 | 1.57 | - | - |
| 15 | Limonene | C5′ | 1036 | 3.86 | 1.97 | 3.31 | 2.27 | 3.74 | 2.91 |
| 16 | Benzyl alcohol | C6′ | 1040 | 0.63 | 0.96 | 2.55 | 1.12 | 0.16 | 0.55 |
| 17 | (Z)-β-ocymene | 1043 | 0.03 | - | - | - | - | - | |
| 18 | Phenylacetaldehyde | 1051 | 0.03 | - | - | - | - | 0.02 | |
| 19 | (E)-β-ocymene | 1054 | 0.02 | - | 0.11 | - | - | - | |
| 20 | γ-Terpinene | C7′ | 1066 | 2.53 | 0.11 | 8.96 | 4.49 | - | - |
| 21 | Octan-1-ol | C8′ | 1075 | 1.17 | 1.33 | 2.46 | 2.24 | 0.12 | 0.16 |
| 22 | α-Terpinolene | 1093 | 0.11 | 0.07 | 0.19 | 0.14 | 0.01 | - | |
| 23 | Linalool | 1103 | - | 0.86 | 0.25 | 0.22 | - | - | |
| 24 | Nonanal | 1107 | 0.03 | - | - | - | - | - | |
| 25 | Benzeneethanol | 1117 | 0.02 | - | - | - | - | - | |
| 26 | trans-Pinocarveol 3 | 1145 | - | 0.05 | 0.35 | - | - | - | |
| 27 | Pinocarvone | 1169 | 0.10 | 0.04 | 0.26 | - | - | - | |
| 28 | 4-Terpineol | 1182 | 0.06 | 0.03 | 0.35 | 0.15 | - | - | |
| 29 | α-Terpineol | 1195 | 0.22 | 0.58 | 0.41 | - | - | - | |
| 30 | Myrtenal | 1199 | 0.15 | 0.07 | 0.70 | 0.11 | 0.02 | - | |
| 31 | Decanal | 1208 | - | - | 0.19 | 0.11 | - | - | |
| 32 | Verbenone | 1212 | 0.04 | - | 0.10 | - | - | - | |
| 33 | 2-Phenoxyethanol | 1223 | 0.01 | - | - | - | - | - | |
| 34 | Bicycloelemene | C9′ | 1342 | 2.18 | 2.40 | 0.43 | 1.73 | 0.34 | 0.68 |
| 35 | α-Cubebene | 1353 | - | - | - | - | 0.04 | 0.05 | |
| 36 | α-Copaene | C10′ | 1375 | 0.07 | - | - | - | 1.33 | 1.55 |
| 37 | α-Gurjunene | 1412 | - | - | 0.79 | - | - | - | |
| 38 | trans-Caryophyllene | C11′ | 1423 | 0.54 | 0.91 | 6.35 | 2.30 | 15.49 | 6.65 |
| 39 | Aromadendrene | 1438 | 0.25 | - | - | - | - | - | |
| 40 | Alloaromadendrene | 1443 | - | - | - | 0.25 | 0.06 | 0.14 | |
| 41 | Selina-5,11-diene 3 | 1447 | - | 0.26 | - | - | - | - | |
| 42 | γ-Muurolene | 1455 | - | - | - | - | 0.09 | 0.32 | |
| 43 | trans-β-Farnesene | C12′ | 1461 | 4.88 | 7.71 | 3.55 | 4.90 | 4.02 | 2.17 |
| 44 | Isocaryophyllene | C13′ | 1471 | 7.10 | 6.85 | - | 5.17 | - | - |
| 45 | α-Amorphene | 1480 | - | - | - | - | 0.19 | - | |
| 46 | Germacrene D | C14′ | 1484 | 12.18 | 2.16 | 1.77 | 1.22 | 53.63 | 51.08 |
| 47 | trans-β-Ionone | 1489 | - | 0.27 | 0.39 | 0.16 | - | - | |
| 48 | β-Cadinene | 1494 | - | - | - | - | 0.01 | 0.01 | |
| 49 | Bicyclogermacrene | C15′ | 1498 | 47.32 | 57.50 | 10.21 | 48.08 | 9.54 | 18.03 |
| 50 | α-Muurolene | 1502 | - | - | - | - | 0.07 | - | |
| 51 | γ-Cadinene | 1517 | - | - | - | - | 0.25 | 0.32 | |
| 52 | δ-Cadinene | C16′ | 1527 | 0.10 | - | - | - | 0.91 | 1.32 |
| 53 | Dihydroactinidiolide | 1532 | 0.17 | 0.26 | 0.42 | 0.22 | 0.12 | 0.20 | |
| 54 | Cadina-1,4-diene | 1536 | - | - | - | - | - | 0.05 | |
| 55 | Spathulenol | 1580 | - | - | - | - | 0.34 | - | |
| 56 | Viridiflorol | C17′ | 1595 | - | - | 24.19 | - | - | - |
| Total identified [%] | 95.99 | 97.09 | 93.06 | 92.99 | 94.58 | 89.35 | |||
| No. | Compound | CD 1(A 2/B 3) | S. romana K (A 2/B 3) | S. romana B (A 2/B 3) | S. romana M (A 2/B 3) | S. romana P (A 2/B 3) | S. montana J (A 2/B 3) | S. montana M (A 2/B 3) |
|---|---|---|---|---|---|---|---|---|
| 1 | Acetic acid | C1/- | -/- | 0.85/- | 3.11/- | 1.33/- | 0.32/- | 0.55/- |
| 2 | α-Thujene | C2/- | 0.47/0.55 | 1.03/0.41 | 1.08/0.79 | 0.21/0.58 | 0.16/0.07 | 0.05/0.04 |
| 3 | α-Pinene | C3/C1′ | 1.32/2.09 | 0.94/2.31 | 2.51/3.91 | 2.19/2.68 | 3.42/1.69 | 1.23/1.14 |
| 4 | Benzaldehyde | C4/- | 0.44/0.06 | 0.32/0.08 | 2.58/0.40 | 0.71/0.15 | 0.37/0.04 | 0.42/0.10 |
| 5 | β-Pinene | C5/C2′ | 3.69/6.78 | 2.61/8.01 | 6.67/13.26 | 8.01/10.35 | 2.81/1.80 | 1.52/1.46 |
| 6 | β-Myrcene | C6/- | 0.80/0.55 | 0.84/0.40 | 1.27/0.99 | 0.88/0.80 | 0.91/0.28 | 0.64/0.20 |
| 7 | α-Phellandrene | C7/- | 0.32/0.15 | 3.37/0.83 | 2.54/0.27 | 0.20/0.22 | 0.10/0.02 | 0.09/- |
| 8 | α-Terpinene | C8/C3′ | 0.62/0.53 | 0.61/- | 1.32/1.15 | 0.38/1.01 | 0.07/- | 0.07/- |
| 9 | p-Cymene | C9/C4′ | 0.99/0.81 | 1.81/0.16 | 5.49/3.10 | 1.32/1.57 | 0.04/- | 0.05/- |
| 10 | Limonene | C10/C5′ | 6.34/3.86 | 7.58/1.97 | 7.10/3.31 | 1.68/2.27 | 8.14/3.74 | 6.31/2.91 |
| 11 | Benzyl alcohol | C11/C6′ | 2.96/0.63 | 3.82/0.96 | 13.28/2.55 | 3.84/1.12 | 0.84/0.16 | 2.14/0.55 |
| 12 | γ-Terpinene | C12/C7′ | 1.75/2.53 | 0.98/0.11 | 3.67/8.96 | 1.40/4.49 | 0.08/- | 0.08/- |
| 13 | Octan-1-ol | C13/C8′ | 1.37/1.17 | 2.22/1.33 | 3.55/2.46 | 2.50/2.24 | 0.35/0.12 | 0.29/0.16 |
| 14 | α-Terpineol | C14/- | 0.50/0.22 | 3.85/0.58 | 1.25/0.41 | 0.02/- | 0.04/- | -/- |
| 15 | Myrtenal | C15/- | 0.39/0.15 | 0.54/0.07 | 1.03/0.70 | 0.47/0.11 | -/0.02 | -/- |
| 16 | Bicycloelemene | -/C9′ | 0.75/2.18 | 0.53/2.40 | -/0.43 | 0.40/1.73 | 0.22/0.34 | 0.41/0.68 |
| 17 | α-Copaene | C16/C10′ | 0.18/0.07 | -/- | -/- | -/- | 1.67/1.33 | 2.02/1.55 |
| 18 | α-Gurjunene | C17/- | 1.17/- | 0.95/- | 0.61/0.79 | 0.85/- | 0.12/- | 0.41/- |
| 19 | Aristolene | C18/- | 1.08/- | 0.91/- | -/- | -/- | -/- | -/- |
| 20 | trans-Caryophyllene | C19/C11′ | -/0.54 | -/0.91 | 3.60/6.35 | 23.88/2.30 | 11.89/15.49 | 6.56/6.65 |
| 21 | Aromadendrene | C20/- | 1.11/0.25 | 1.05/- | -/- | 0.96/- | 0.23/- | 2.87/- |
| 22 | Alloaromadendrene | C21/- | 7.32/- | 6.69/- | 2.35/- | 6.27/0.25 | 0.76/0.06 | 0.90/0.14 |
| 23 | Selina-5,11-diene 4 | C22/- | 2.52/- | 2.28/0.26 | -/- | 1.91/- | -/- | -/- |
| 24 | trans-β-Farnesene | C23/C12′ | 5.12/4.88 | 12.79/7.71 | 1.00/3.55 | 5.58/4.90 | 4.83/4.02 | 3.56/2.17 |
| 25 | Isocaryophyllene | C24/C13′ | 4.70/7.10 | 2.39/6.85 | -/- | 3.22/5.17 | -/- | -/- |
| 26 | α-Amorphene | C25/- | 0.58/- | -/- | -/- | -/- | 2.84/0.19 | 3.31/- |
| 27 | Germacrene D | C26/C14′ | 1.50/12.18 | 0.06/2.16 | -/1.77 | -/1.22 | 23.23/53.63 | 17.04/51.08 |
| 28 | β-Cadinene | C27/- | 2.19/- | 1.68/- | -/- | 1.24/- | 0.98/0.01 | 1.47/0.01 |
| 29 | Ledene | C28-/ | -/- | -/- | 8.20/- | -/- | 2.27/- | -/- |
| 30 | Bicyclogermacrene | C29/C15′ | 23.04/47.32 | 20.48/57.50 | -/10.21 | 15.16/48.08 | 4.19/9.54 | 11.76/18.03 |
| 31 | α-Muurolene | C30/- | 0.61/- | 0.18/- | -/- | -/- | 1.78/0.07 | 2.93/- |
| 32 | γ-Cadinene | C31/- | 0.58/- | -/- | -/- | -/- | 3.33/0.25 | 3.37/0.32 |
| 33 | δ-Cadinene | C32/C16′ | 1.84/0.10 | 0.15/- | -/- | -/- | 7.57/0.91 | 8.85/1.32 |
| 34 | Cadina-1,4-diene | C33/- | 0.20/- | -/- | -/- | -/- | 0.94/- | 1.24/0.05 |
| 35 | α-Cadinene | C34/- | 0.24/- | -/- | -/- | -/- | 1.41/- | 1.70/- |
| 36 | α-Calacorene | C35/- | 0.47/- | 0.04/- | -/- | -/- | 1.05/- | 1.36/- |
| 37 | Spathulenol | C36/- | 0.58/- | 1.30/- | -/- | 0.52/- | 0.15/0.34 | 0.47/- |
| 38 | Viridiflorol | C37/C17′ | 0.42/- | 0.36/- | 10.29/24.19 | 0.20/- | 0.08/- | 0.21/- |
| Species | Sample Code | Voucher No. | Location | Date of Collection | Latitude | Longitude | Köppen Climate Classification 1 |
|---|---|---|---|---|---|---|---|
| S. romana | S. romana K | 18 021 | Istria, Kamenjak | 13 June 2020 | 44°47′46.15″ N | 13°54′25.96″ E | Cfa 2 |
| S. romana B | 18 023 | Dalmatia, Blato | 21 June 2020 | 43°10′12.71″ N | 17°11′45.75″ E | Csa 3 | |
| S. romana M | 18 020 | Dalmatia, Morinje Bay | 7 June 2020 | 43°40′44.79″ N | 15°57′41.25″ E | Csa 3 | |
| S. romana P | 18 022 | Dalmatia, Brač, Pražnica | 20 June 2020 | 43°19′1.3″ N | 16°40′24.45″ E | Csb 4 | |
| S. montana | S. montana J | 18 010 | Dalmatia, Ježević | 6 June 2020 | 43°55′1.51″ N | 16°28′8.41″ E | Cfb 5 |
| S. montana M | 18 011 | Dalmatia, Mosor, Gornje Sitno | 22 June 2020 | 43°31′13.56″ N | 16°36′10.44″ E | Cfa 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marić, T.; Friščić, M.; Marijanović, Z.; Maleš, Ž.; Jerković, I. Comparison of Volatile Organic Compounds of Sideritis romana L. and Sideritis montana L. from Croatia. Molecules 2021, 26, 5968. https://doi.org/10.3390/molecules26195968
Marić T, Friščić M, Marijanović Z, Maleš Ž, Jerković I. Comparison of Volatile Organic Compounds of Sideritis romana L. and Sideritis montana L. from Croatia. Molecules. 2021; 26(19):5968. https://doi.org/10.3390/molecules26195968
Chicago/Turabian StyleMarić, Tihana, Maja Friščić, Zvonimir Marijanović, Željan Maleš, and Igor Jerković. 2021. "Comparison of Volatile Organic Compounds of Sideritis romana L. and Sideritis montana L. from Croatia" Molecules 26, no. 19: 5968. https://doi.org/10.3390/molecules26195968