Biological Activities of Paeonol in Cardiovascular Diseases: A Review
Abstract
:1. Introduction
2. Pharmacological Features of Paeonol
2.1. Bioactive Compounds in Cortex Moutan
2.2. Pharmacokinetics and Drug Delivery of Paeonol
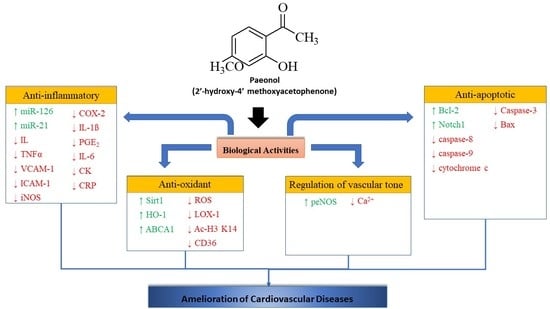
3. Mechanism of Action of Paeonol in Cardiovascular Diseases
3.1. Anti-Oxidant Mechanism
3.2. Anti-Inflammatory Mechanism
3.3. Regulation of Vascular Tone
3.4. Anti-Apoptotic Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ac-H3 K14 | histone H3 acetylation at lysine 14 |
| Ac-H4 K16 | histone H4 acetylation at lysine 16 |
| AIF | apoptosis inducing factor |
| ApoE−/− | apoliprotein E-deficient |
| BrdU | Bromodeoxyuridine/5-bromo-2’-deoxyuridine |
| CD36 | cluster of differentiation 36 |
| c-Jun-AP-1 | c-JUN-Activator protein-1 |
| CK | creatinine kinase |
| CK-MB | creatine kinase-MB |
| EDCF | endothelium-derived contracting factors |
| EDHF | endothelium-derived hyperpolarizing factor |
| EDRFs | endothelium-derived relaxing factors |
| eNOS | endothelial nitric oxide synthase |
| HUVECs | human umbilical vascular endothelial cells |
| LOX-1 | lipoprotein receptor-1 |
| LTT assay | lymphocyte transformation test assay |
| LVEDD | left ventricular end-diastolic dimension |
| MCP-1 | monocyte chemoattractant protein-1 |
| PRRs | pattern recognition receptors |
| RAECs | rat aortic endothelial cells |
| SA-beta-galactosidase | Senescence-associated beta-galactosidase |
| sGC | soluble guanylyl cyclase |
| SIRT 1 | Sirtuin 1 |
| STZ | streptozotocin |
| TUNEL | deoxynucleotidyl transferase-mediated dUTP nick-end labelling |
| VECs | vascular endothelial cells |
References
- Clark, H. NCDs: A challenge to sustainable human development. Lancet 2013, 381, 510–511. [Google Scholar] [CrossRef]
- Middlemiss, D.; Watson, S.P. A medicinal chemistry case study: An account of an angiotensin II antagonist drug discovery programme. Tetrahedron 1994, 50, 13049–13080. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Estimates 2016 Summary Tables: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. The Global Health Observatory; United Nations: Geneva, Switzerland, 2018. [Google Scholar]
- Alwan, A. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Bonita, R.; Magnusson, R.; Bovet, P.; Zhao, D.; Malta, D.C.; Geneau, R.; Suh, I.; Thankappan, K.R.; McKee, M.; Hospedales, J.; et al. Country actions to meet UN commitments on non-communicable diseases: A stepwise approach. Lancet 2013, 381, 575–584. [Google Scholar] [CrossRef]
- Sacco, R.L.; Roth, G.A.; Reddy, K.S.; Arnett, D.K.; Bonita, R.; Gaziano, T.A.; Heidenreich, P.A.; Huffman, M.; Mayosi, B.M.; Mendis, S.; et al. The heart of 25 by 25: Achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke. Circulation 2016, 133, e674–e690. [Google Scholar] [CrossRef]
- Pashkow, F.J. Oxidative stress and inflammation in heart disease: Do antioxidants have a role in treatment and/or prevention? Int. J. Inflamm. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hajjar, D.P.; Gotto, A.M. Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. Am. J. Pathol. 2013, 182, 1474–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Waltenberger, B.; Mocan, A.; Šmejkal, K.; Heiss, E.H.; Atanasov, A.G. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules 2016, 21, 807. [Google Scholar] [CrossRef]
- Adki, K.M.; Kulkarni, Y.A. Chemistry, pharmacokinetics, pharmacology and recent novel drug delivery systems of paeonol. Life Sci. 2020, 250, 117544. [Google Scholar] [CrossRef] [PubMed]
- Gjertsen, F.B.; Solheim, E.; Scheline, R.R. Metabolism of aromatic plant ketones in rats: Acetovanillone and paeonol. Xenobiotica 1988, 18, 225–234. [Google Scholar] [CrossRef]
- Li, Y.-J.; Bao, J.-X.; Xu, J.-W.; Murad, F.; Bian, K. Vascular dilation by paeonol—A mechanism study. Vasc. Pharmacol. 2010, 53, 169–176. [Google Scholar] [CrossRef]
- Hirai, A.; Terano, T.; Hamazaki, T.; Sajiki, J.; Saito, H.; Tahara, K.; Tamura, Y.; Kumagai, A. Studies on the mechanism of antiaggregatory effect of moutan cortex. Thromb. Res. 1983, 31, 29–40. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Ge, N.; Zhang, Z.Y. Theoretical elucidation of activity differences of five phenolic antioxidants. Zhongguo Yao Li Xue Bao Acta Pharmacol. Sin. 1999, 20, 363–366. [Google Scholar]
- Nizamutdinova, I.T.; Jin, Y.C.; Kim, J.S.; Yean, M.H.; Kang, S.S.; Kim, Y.S.; Lee, J.H.; Seo, H.G.; Kim, H.J.; Chang, K.C. Paeonol and paeoniflorin, the main active principles of paeonia albiflora, protect the heart from myocardial ischemia/reperfusion injury in rats. Planta Med. 2008, 74, 14–18. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Bates, S.; Gurney, A.M. The effects of paeonol on the electrophysiological properties of cardiac ventricular myocytes. Eur. J. Pharmacol. 2006, 545, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Qin, Y.; Chen, C.; Guo, X. Beneficial effects exerted by paeonol in the management of atherosclerosis. Oxidative Med. Cell. Longev. 2018, 2018, 1098617. [Google Scholar] [CrossRef]
- Li, S.-S.; Wu, Q.; Yin, D.-D.; Feng, C.; Liu, Z.-A.; Wang, L.-S. Phytochemical variation among the traditional Chinese medicine Mu Dan Pi from Paeonia suffruticosa (tree peony). Phytochemistry 2018, 146, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Liu, J.-L.; Wang, J.-K. Synthesis and anti-tumor activity of paeonol and its derivatives. Yao Xue Xue Bao Acta Pharm. Sin. 2012, 47, 72–76. [Google Scholar]
- Jung, E.-H.; Hwang, J.-S.; Kwon, M.-Y.; Kim, K.-H.; Cho, H.; Lyoo, I.K.; Shin, S.; Park, J.-H.; Han, I.-O. A tryptamine-paeonol hybridization compound inhibits LPS-mediated inflammation in BV2 cells. Neurochem. Int. 2016, 100, 35–43. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, B.; Yang, Y.; Gong, X.; Chen, Z.; Wang, Z.; Zhang, P.; Zhang, Q. Synthesis and anti-inflammatory activity of paeonol analogues in the murine model of complete Freund’s adjuvant induced arthritis. Bioorg. Med. Chem. Lett. 2016, 26, 5218–5221. [Google Scholar] [CrossRef]
- Fu, P.-K.; Yang, C.-Y.; Huang, S.-C.; Hung, Y.-W.; Jeng, K.-C.; Huang, Y.-P.; Chuang, H.; Huang, N.-C.; Li, J.-P.; Hsu, M.-H.; et al. Evaluation of LPS-induced acute lung injury attenuation in rats by aminothiazole-paeonol derivatives. Molecules 2017, 22, 1605. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.-H.; Cao, S.-W.; Yu, Y.-Y. Synthesis, characterization and biological evaluation of paeonol thiosemicarbazone analogues as mushroom tyrosinase inhibitors. Int. J. Biol. Macromol. 2013, 62, 589–595. [Google Scholar] [CrossRef]
- Han, F.; Zhuang, T.-T.; Chen, J.-J.; Zhu, X.-L.; Cai, Y.-F.; Lü, Y.-P. Novel derivative of Paeonol, Paeononlsilatie sodium, alleviates behavioral damage and hippocampal dendritic injury in Alzheimer’s disease concurrent with cofilin1/phosphorylated-cofilin1 and RAC1/CDC42 alterations in rats. PLoS ONE 2017, 12, e0185102. [Google Scholar] [CrossRef] [Green Version]
- Zhou, A.; Wu, H.; Pan, J.; Wang, X.; Li, J.; Wu, Z.; Hui, A. Synthesis and evaluation of paeonol derivatives as potential multifunctional agents for the treatment of alzheimer’s disease. Molecules 2015, 20, 1304–1318. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-Y.; Kapoor, M.; Huang, Y.-P.; Lin, H.-H.; Liang, Y.-C.; Lin, Y.-L.; Huang, S.-C.; Liao, W.-N.; Chen, J.-K.; Huang, J.-S.; et al. Synthesis and evaluation of aminothiazole-paeonol derivatives as potential anticancer agents. Molecules 2016, 21, 145. [Google Scholar] [CrossRef] [Green Version]
- Anh, H.L.T.; Cuc, N.T.; Tai, B.H.; Yen, P.H.; Nhiem, N.X.; Thao, D.T.; Nam, N.H.; Van Minh, C.; Van Kiem, P.; Kim, Y.H. Synthesis of chromonylthiazolidines and their cytotoxicity to human cancer cell lines. Molecules 2015, 20, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-J.; Chuang, H.; Liang, Y.-C.; Lin, H.-H.; Horng, J.-C.; Kuo, Y.-C.; Chen, C.-W.; Tsai, F.-Y.; Yen, S.-C.; Chou, S.-C.; et al. Design, synthesis, and bioevaluation of paeonol derivatives as potential anti-HBV agents. Eur. J. Med. Chem. 2015, 90, 428–435. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Hsu, M.-H.; Huang, P.-H.; Hsieh, C.-T.; Chiu, Y.-M.; Shieh, D.-C.; Lee, Y.-J.; Tsay, G.J.; Wu, Y.-Y. A paeonol derivative, YPH-PA3 promotes the differentiation of monocyte/macrophage lineage precursor cells into osteoblasts and enhances their autophagy. Eur. J. Pharmacol. 2018, 832, 104–113. [Google Scholar] [CrossRef]
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Du, S.; Xu, B.; Wang, S.; Zhai, Y.; Song, X.; Li, P. In Situ and In Vivo study of nasal absorption of paeonol in rats. Int. J. Mol. Sci. 2010, 11, 4882–4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Xie, Y.-H.; Yang, Q.; Wang, S.-W.; Zhang, B.-L.; Wang, J.-B.; Cao, W.; Bi, L.-L.; Sun, J.-Y.; Miao, S.; et al. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS ONE 2012, 7, e48872. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, S.; Zhang, B.-L.; Xie, Y.; Wang, J.; Yang, Q.; Cao, W.; Hu, J.; Duan, L. Influence of Co-Administered Danshensu on Pharmacokinetic Fate and Tissue Distribution of Paeonol in Rats. Planta Medica 2011, 78, 135–140. [Google Scholar] [CrossRef]
- Li, S.-S.; Li, G.-F.; Liu, L.; Li, H.; Jiang, X.; Li, X.-L.; Liu, Z.-G.; Zuo, T.; Weng, L.-D.; Liu, Q. Optimization of paeonol-loaded microparticle formulation by response surface methodology. J. Microencapsul. 2015, 32, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Luo, M.; Chen, J. Transdermal delivery of paeonol using cubic gel and microemulsion gel. Int. J. Nanomed. 2011, 6, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Li, B.; Liu, T.; Wang, X.; Zhu, Y.; Wang, L.; Niu, X.; Xiao, Y.; Sun, Q. Evaluation of paeonol-loaded transethosomes as transdermal delivery carriers. Eur. J. Pharm. Sci. 2017, 99, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Li, G.-F.; Liu, L.; Jiang, X.; Zhang, B.; Liu, Z.-G.; Li, X.-L.; Weng, L.-D.; Zuo, T.; Liu, Q. Evaluation of paeonol skin-target delivery from its microsponge formulation: In Vitro skin permeation and In Vivo microdialysis. PLoS ONE 2013, 8, e79881. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-C.; Zhu, N.; Zhu, J.-X.; Zhang, W.-J.; Zhang, H.-M.; Wang, Q.-Q.; Wu, X.-X.; Wang, X.; Zhang, J.; Hao, J.-F. Self-assembled cubic liquid crystalline nanoparticles for transdermal delivery of paeonol. Med Sci. Monit. 2015, 21, 3298–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Jia, F.; Hou, Z.; Ruan, S.; Lu, Q. Delivery of paeonol by nanoparticles enhances its In Vitro and In Vivo antitumor effects. Int. J. Nanomed. 2017, 12, 6605–6616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Zhang, J.; Wu, L.; Wu, H.; Dai, M. Paeonol nanoemulsion for enhanced oral bioavailability: Optimization and mechanism. Nanomedicine 2018, 13, 269–282. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, Y.; Hu, Q.; Zeng, D.; Hua, F.; Meng, W.; Wang, W.; Bao, G.-H. Optimization of paeonol-loaded poly(butyl-2-cyanoacrylate) nanocapsules by central composite design with response surface methodology together with the antibacterial properties. Eur. J. Pharm. Sci. 2017, 101, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.V.; Padmaja, G.; Kuppusamy, P.; Kutala, V.K. Oxidative stress in cardiovascular disease. NISCAIR Online Period. Repos. 2009, 46, 421–440. [Google Scholar]
- Ding, Z.; Liu, S.; Wang, X.; Khaidakov, M.; Dai, Y.; Mehta, J.L. Oxidant stress in mitochondrial DNA damage, autopha-gy and inflammation in atherosclerosis. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Yon, J.-Y.; Cai, H. Mechanisms and consequences of eNOS dysfunction in hypertension. J. Hypertens. 2015, 33, 1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, E.M.; Degasperi, G.R.; de Oliveira, H.C.; Vercesi, A.E.; de Faria, E.C.; Castilho, L.N. Reactive oxygen spe-cies generation in peripheral blood monocytes and oxidized LDL are increased in hyperlipidemic patients. Clin. Biochem. 2009, 42, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.-h.; Zhang, Y.-w.; Zhou, H.-h. Paeonol suppresses oxidized low-density lipoprotein induced endothelial cell apoptosis via activation of LOX-1/p38MAPK/NF-κB pathway. J. Ethnopharmacol. 2013, 146, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Jamal, J.; Mustafa, M.R.; Wong, P.-F. Paeonol protects against premature senescence in endothelial cells by modulating Sirtuin 1 pathway. J. Ethnopharmacol. 2014, 154, 428–436. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Yu, C.; Yang, H.; Zhang, C.; Ye, Y.; Xiao, S. Paeonol suppresses lipid accumulation in macrophages via upregulation of the ATP-binding cassette transporter A1 and downregulation of the cluster of differentiation. Int. J. Oncol. 2015, 46, 764–774. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Mozos, I.; Malainer, C.; Horbańczuk, J.; Gug, C.; Stoian, D.; Luca, C.T.; Atanasov, A.G. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front. Immunol. 2017, 8, 1058. [Google Scholar] [CrossRef] [Green Version]
- Katsiari, C.G.; Bogdanos, D.P.; I Sakkas, L. Inflammation and cardiovascular disease. World J. Transl. Med. 2019, 8, 1–8. [Google Scholar] [CrossRef]
- Fioranelli, M.; Bottaccioli, A.G.; Bottaccioli, F.; Bianchi, M.; Rovesti, M.; Roccia, M.G. Stress and inflammation in coronary artery disease: A review psychoneuroendocrineimmunology-based. Front. Immunol. 2018, 9, 2031. [Google Scholar] [CrossRef] [PubMed]
- Robbie, L.; Libby, P. Inflammation and atherothrombosis. Ann. N. Y. Acad. Sci. 2006, 947, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, immunity, and infection in atherothrombosis. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Dai, M. Paeonol from Paeonia suffruticosa prevents TNF-α-induced monocytic cell adhesion to rat aortic endothelial cells by suppression of VCAM-1 expression. Phytomedicine 2009, 16, 1027–1032. [Google Scholar] [CrossRef]
- Kim, S.-A.; Lee, H.-J.; Ahn, K.S.; Lee, E.-O.; Ahn, K.-S.; Choi, S.-H.; Jung, S.-J.; Kim, J.Y.; Baek, N.; Kim, S.-H. Paeonol exerts anti-angiogenic and anti-metastatic activities through downmodulation of akt activation and inactivation of matrix metalloproteinases. Biol. Pharm. Bull. 2009, 32, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Chen, J.; Dai, M. Paeonol promotes microRNA-126 expression to inhibit monocyte adhesion to ox-LDL-injured vascular endothelial cells and block the activation of the PI3K/Akt/NF-κB pathway. Int. J. Mol. Med. 2016, 38, 1871–1878. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-R.; Chen, J.-J.; Dai, M. Paeonol protects rat vascular endothelial cells from ox-LDL-induced injury in vitro via downregulating microRNA-21 expression and TNF-α release. Acta Pharmacol. Sin. 2014, 35, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, C.; Wu, H.; Xie, X.; Sun, Y.; Dai, M. Paeonol attenuated inflammatory response of endothelial cells via stimulating monocytes-derived exosomal microRNA-223. Front. Pharmacol. 2018, 9, 1105. [Google Scholar] [CrossRef]
- Ma, L.; Chuang, C.-C.; Weng, W.; Zhao, L.; Zheng, Y.; Zhang, J.; Zuo, L. Paeonol protects rat heart by improving regional blood perfusion during no-reflow. Front. Physiol. 2016, 7, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.-F.; Leu, S.-J.J.; Shyue, S.-K.; Su, K.-H.; Wei, J.; Lee, T.-S. Novel effect of paeonol on the formation of foam cells: Promotion of LXRα-ABCA1–dependent cholesterol efflux in macrophages. Am. J. Chin. Med. 2013, 41, 1079–1096. [Google Scholar] [CrossRef]
- Kietadisorn, R.; Juni, R.P.; Moens, A.L. Tackling endothelial dysfunction by modulating NOS uncoupling: New insights into its pathogenesis and therapeutic possibilities. Am. J. Physiol. Metab. 2012, 302, E481–E495. [Google Scholar] [CrossRef] [Green Version]
- Griffith, T.M.; Lewis, M.J.; Newby, A.C.; Henderson, A.H. Endothelium-derived relaxing factor. J. Am. Coll. Cardiol. 1988, 12, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Gewaltig, M.T. Vasoprotection by nitric oxide: Mechanisms and therapeutic potential. Cardiovasc. Res. 2002, 55, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Endemann, D.H. Endothelial Dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Mollnau, H.; Wendt, M.; Szoöcs, K.; Lasseègue, B.; Schulz, E.; Oelze, M.; Li, H.; Bodenschatz, M.; August, M.; Kleschyov, A.L.; et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ. Res. 2002, 90, e58–e65. [Google Scholar] [CrossRef] [Green Version]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.K.; Warnholtz, A.; et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001, 88, e14–e22. [Google Scholar] [CrossRef]
- Takimoto, E.; Champion, H.C.; Li, M.; Ren, S.; Rodriguez, E.R.; Tavazzi, B.; Lazzarino, G.; Paolocci, N.; Gabrielson, K.L.; Wang, Y.; et al. Oxidant stress from nitric oxide synthase–3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Investig. 2005, 115, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Pryor, W.A.; Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. Cell. Mol. Physiol. 1995, 268, L699–L722. [Google Scholar] [CrossRef] [PubMed]
- Thuillez, C.; Richard, V. Targeting endothelial dysfunction in hypertensive subjects. J. Hum. Hypertens. 2005, 19, S21–S25. [Google Scholar] [CrossRef]
- Goto, H.; Shimada, Y.; Akechi, Y.; Kohta, K.; Hattori, M.; Terasawa, K. Endothelium-dependent vasodilator effect of extract prepared from the roots ofpaeonia lactifloraon isolated rat aorta. Planta Med. 1996, 62, 436–439. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Cao, Y.-X.; Weng, W.-L.; Li, Y.-K.; Zhao, L. Paeonol induces vasodilatation in rat mesenteric artery via inhibiting extracellular Ca2+ influx and intracellular Ca2+ release. Chin. J. Integr. Med. 2013, 19, 510–516. [Google Scholar] [CrossRef]
- Choy, K.-W.; Mustafa, M.R.; Lau, Y.S.; Liu, J.; Murugan, D.; Lau, C.W.; Wang, L.; Zhao, L.; Huang, Y. Paeonol protects against endoplasmic reticulum stress-induced endothelial dysfunction via AMPK/PPARδ signaling pathway. Biochem. Pharmacol. 2016, 116, 51–62. [Google Scholar] [CrossRef]
- Choy, K.W.; Lau, Y.S.; Murugan, D.; Mustafa, M.R. Chronic treatment with paeonol improves endothelial function in mice through inhibition of endoplasmic reticulum stress-mediated oxidative stress. PLoS ONE 2017, 12, e0178365. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Goldblum, S.E. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am. J. Physiol. Cell. Mol. Physiol. 2003, 284, L899–L914. [Google Scholar] [CrossRef] [Green Version]
- Mann, U.L.; Topkara, V.K.; Evans, S.; Barger, P.M. Innate immunity in the adult mammalian heart: For whom the cell tolls. Trans. Am. Clin. Clim. Assoc. 2010, 121, 34–51. [Google Scholar]
- Choy, K.W.; Lau, Y.S.; Murugan, D.; Vanhoutte, P.M.; Mustafa, M.R. Paeonol attenuates LPS-induced endothelial dysfunction and apoptosis by inhibiting BMP4 and TLR4 signaling simultaneously but independently. J. Pharmacol. Exp. Ther. 2018, 364, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Gai, Z.; Wang, Z.; Zhang, L.; Ma, J.; Zhu, Q. Paeonol protects against hypertension in spontaneously hypertensive rats by restoring vascular endothelium. Biosci. Biotechnol. Biochem. 2019, 83, 1992–1999. [Google Scholar] [CrossRef]
- Teringova, E.; Tousek, P. Apoptosis in ischemic heart disease. J. Transl. Med. 2017, 15, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tower, J. Programmed cell death in aging. Ageing Res. Rev. 2015, 23, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraste, A.; Pulkki, K.; Kallajoki, M.; Henriksen, K.; Parvinen, M.; Voipio-Pulkki, L.-M. Apoptosis in human acute myocardial infarction. Circulation 1997, 95, 320–323. [Google Scholar] [CrossRef]
- Olivetti, G. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J. Mol. Cell. Cardiol. 1996, 28, 2005–2016. [Google Scholar] [CrossRef]
- Abbate, A.; Biondi-Zoccai, G.G.; Bussani, R.; Dobrina, A.; Camilot, D.; Feroce, F.; Rossiello, R.; Baldi, F.; Silvestri, F.; Biasucci, L.M.; et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J. Am. Coll. Cardiol. 2003, 41, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Stoneman, V.E.A.; Bennett, M. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin. Sci. 2004, 107, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.-H.; Kang, P.M. Apoptosis in cardiovascular diseases: Mechanism and clinical implications. Korean Circ. J. 2010, 40, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Kang, P.M.; Haunstetter, A.; Aoki, H.; Usheva, A.; Izumo, S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ. Res. 2000, 87, 118–125. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Gores, G.J. Life and death by death receptors. FASEB J. 2009, 23, 1625–1637. [Google Scholar] [CrossRef] [Green Version]
- Chiong, M.; Wang, Z.; Pedrozo, Z.; Cao, D.J.; Troncoso, R.; Ibacache, M.; Criollo, A.; Nemchenko, A.; A Hill, J.; Lavandero, S. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis. 2011, 2, e244. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, K.; Whelan, R.S.; Kitsis, R.N. Mechanisms of cell death in heart disease. Arter. Thromb. Vasc. Biol. 2012, 32, 1552–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín-García, J. Cell death in the pathogenesis and progression of heart failure. Hear. Fail. Rev. 2016, 21, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Bao, Q.; Shi, Y. Apoptosome: A platform for the activation of initiator caspases. Cell Death Differ. 2006, 14, 56–65. [Google Scholar] [CrossRef]
- Nicholson, D.W.; Thornberry, N.A. Caspases: Killer proteases. Trends Biochem. Sci. 1997, 22, 299–306. [Google Scholar] [CrossRef]
- Cregan, S.P.; Dawson, V.; Slack, R.S. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 2004, 23, 2785–2796. [Google Scholar] [CrossRef] [Green Version]
- Penninger, J.; Kroemer, G. Mitochondria, AIF and caspases—Rivaling for cell death execution. Nat. Cell Biol. 2003, 5, 97–99. [Google Scholar] [CrossRef]
- Florescu, M.; Magda, L.S.; Enescu, O.A.; Jinga, D.; Vinereanu, D. Early detection of epirubicin-induced cardiotoxicity in patients with breast cancer. J. Am. Soc. Echocardiogr. 2014, 27, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Khasraw, M.; Bell, R.; Dang, C. Epirubicin: Is it like doxorubicin in breast cancer? A clinical review. Breast 2012, 21, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, C.; Wang, R.; Li, J.; Zhou, M.; Yan, M.; Xue, X.; Wang, C. Cardioprotective effect of paeonol against epirubicin-induced heart injury via regulating miR-1 and PI3K/AKT pathway. Chem. Interact. 2018, 286, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Song, A.; Hu, W.; Dai, M. The anti-atherosclerotic effect of paeonol against vascular smooth muscle cell proliferation by up-regulation of autophagy via the AMPK/mTOR signaling pathway. Front. Pharmacol. 2018, 8, 948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heusch, G. Treatment of myocardial ischemia/reperfusion injury by ischemic and pharmacological postconditioning. Compr. Physiol. 2015, 5, 1123–1145. [Google Scholar] [CrossRef] [PubMed]
- Von Elverfeldt, D.; Maier, A.; Duerschmied, D.; Braig, M.; Witsch, T.; Wang, X.; Mauler, M.; Neudorfer, I.; Menza, M.; Idzko, M.; et al. Dual-contrast molecular imaging allows noninvasive characterization of myocardial ischemia/reperfusion injury after coronary vessel occlusion in mice by magnetic resonance imaging. Circulation 2014, 130, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Du, J.; Song, X.; He, L.; Zhang, Y.; Li, X.; Qiu, C.; Zhang, Y.; Hou, J.; Feng, J.; et al. Melatonin prevents adverse myocardial infarction remodeling via Notch1/Mfn2 pathway. Free. Radic. Biol. Med. 2016, 97, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Fang, Y.H.; Zhao, Y.; Zou, B.; Xu, Q.R.; Xu, H.; Rao, C.X.; Liu, J.C. The effect of Notch1 on myocardial ischemia reperfusion injury. Zhonghua Yi Xue Za Zhi 2016, 96, 1591–1596. [Google Scholar]
- Li, X.; Yang, R.; Yang, X.; Zheng, X. Paeonol protects H9C2 cardiomyocytes from ischemia/reperfusion injury by activating Notch1 signaling pathway In Vitro. Int. J. Clin. Exp. Med. 2017, 10, 2866–2873. [Google Scholar]
- Cusack, M. Recent advances in ischaemic heart disease. Postgrad. Med J. 2000, 76, 542–546. [Google Scholar] [CrossRef]
- Lin-Rui, D.; Song, F.; Duan, L.-R.; Sheng, J.-J.; Xie, Y.-H.; Yang, Q.; Chen, Y.; Dong, Q.-Q.; Zhang, B.-L.; Wang, S.-W. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci. Rep. 2016, 6, 23693. [Google Scholar] [CrossRef]
- Sun, D.; Shen, M.; Li, J.; Li, W.; Zhang, Y.; Zhao, L.; Zhang, Z.; Yuan, Y.; Wang, H.; Cao, F. Cardioprotective effects of tanshinone IIA pretreatment via kinin B2 receptor-Akt-GSK-3beta dependent pathway in experimental diabetic cardiomyopathy. Cardiovasc. Diabetol. 2011, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frustaci, A.; Kajstura, J.; Chimenti, C.; Jakoniuk, I.; Leri, A.; Maseri, A.; Nadal-Ginard, B.; Anversa, P. Myocardial cell death in human diabetes. Circ. Res. 2000, 87, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Z.-Q.; Bao, L.; Li, Z.-C.; Qu, J.-X. Protective effects of paeonol on cardiovascular complications in diabetes mellitus involves modulation of PI3K /Akt-GSK-3β signalling, regulation of protease-activated receptor-1 expressions and down-regulation of inflammatory mediators. Bangladesh J. Pharmacol. 2015, 10, 903–916. [Google Scholar] [CrossRef] [Green Version]

| Effects | Models | Mechanism | Reference |
|---|---|---|---|
| Anti-oxidative effect | Oxidized low-density lipoprotein induced HUVEC apoptosis | ↓ LOX-1, ↓ P38 MAPK, ↓ NF-κB, and ↓ Caspase-3 | [48] |
| Paeonol protects against premature senescence in HUVEC cells | ↓ P53, ↓ Ac-H3 K14, ↓ Ac-H4 K16 and ↓ SA-beta-galactosidase ↑ Sirt1, ↑ BrdU and ↑ Cell growth profile | [50] | |
| Paeonol attenuated intracellular lipid accumulation in RAW264.7 macrophages and ApoE−/− mice | ↓ HO-1, ↓ CD36, ↓ c-Jun-AP-1, and ↓ Calpain ↑ ABCA1 and ↑ Wogonin | [51] | |
| Anti-inflammatory | Rat aortic endothelial cells (RAECs). | ↓ TNF-α-induced ↓ monocytic cell adhesion to rat aortic endothelial cells by of VCAM-1 expression ↓ VCAM1 viaregulation of ERK ½ and p38 | [58] |
| Rat model of carrageenan-evoked thermal hyperalgesia, bFGF stimulated HUVECs | ↓ TNFα, ↓ IL-1ß, ↓ iNOS, ↓ COX-2 and ↓ PGE2 ↓ essential angiogenesis pathways such as proliferation and migration in FGF | [59] | |
| Ox-LDL stimulated VECs isolated from rat thoracic aorta | ↑ miR 126 expression to monocyte adhesion ↓ PI3K/Akt/NF-κB signalling pathway | [60] | |
| Ox-LDL stimulated VECs isolated from rat thoracic aorta | ↑ survival rate of ox-LDL treated VECs ↓ Release of ox-LDL induced TNF-α reverse the PTEN expression | [61] | |
| High fat diet ApoE−/− mice & HUVECs | ↑ miR 223 expression ↓ STAT3 pathway ↓ IL-1ß and IL6 ↓ VCAM 1 and ICAM 1 | [62] | |
| Wistar rats with ischemic reperfusion (IR) injury | ↓ No-reflow area ↓ myocardial ischemic damage ↓ CK, CRP, LDH, TnT levels | [63] | |
| ApoE−/− mice | ↑ LXRα-ABCA1–dependent cholesterol efflux | [64] | |
| Regulation of vascular tone | Spontaneously Hypertensive rats | ↓ elevated blood pressure and increased the cerebral blood flow velocity ↓ vascular endothelium injury | [83] |
| Isolated SD rat aorta preparation | NO dependent vasodilator effects (-) voltage-dependent and receptor-operated Ca2+ channel, as well as intracellular Ca2+ release | [13,76] | |
| Tunicamycin-induced endothelial dysfunction in mice and HUVECs | ↑ AMPK, ↑ PPARγ, ↑ peNOS and ↑NO ↓ AT6, ↓ GRP78, ↓ peIF2α and ↓ ROS, | [78,79] | |
| LPS-induced endothelial dysfunction in mice and HUVECs | ↓ TLR4, ↓ BMP4, ↓ ROS, ↓ p38 and ↓ iNOS, ↑ peNOS and ↑ NO | [82] | |
| Anti-apoptosis effect | Epirubicin-induced Cardiotoxicity in rat cardiac myocytes H9C2 and mice cardiomyocytes LH-1 cell death | ↓ Caspase 3, ↓ Bax and ↓ PI3K/AKT/mTOR ↑ Bcl-2 | [104] |
| I/R-induced apoptosis in H9c2 embryonic rat myocardium-derived cells | ↓ Caspase 3, Bax ↑ Bcl-2, Notch1 | [110] | |
| (ISO)-induced myocardial infarction in rats | ↓ Fas, ↓ TNF-α, ↓ Bax, ↓ caspase-3, ↓ caspase-8, ↓ caspase-9, ↓ cytochrome c, ↓ CK-MB, ↓ cTnI and ↓ cTnT ↑ Bcl-2/Nrf2/PI3K/Akt | [112] | |
| Streptozotocin-induced diabetic cardiomyopathy model in rat | ↓ caspase-3, ↓ TNF-α, ↓ IL-6, ↓ NF-κB, ↓ p65 and ↓ p-Iκ-Bα ↑ PI3K/Akt-GSK-3β | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellasamy, S.; Murugan, D.; Abas, R.; Alias, A.; Seng, W.Y.; Woon, C.K. Biological Activities of Paeonol in Cardiovascular Diseases: A Review. Molecules 2021, 26, 4976. https://doi.org/10.3390/molecules26164976
Vellasamy S, Murugan D, Abas R, Alias A, Seng WY, Woon CK. Biological Activities of Paeonol in Cardiovascular Diseases: A Review. Molecules. 2021; 26(16):4976. https://doi.org/10.3390/molecules26164976
Chicago/Turabian StyleVellasamy, Shalini, Dharmani Murugan, Razif Abas, Aspalilah Alias, Wu Yuan Seng, and Choy Ker Woon. 2021. "Biological Activities of Paeonol in Cardiovascular Diseases: A Review" Molecules 26, no. 16: 4976. https://doi.org/10.3390/molecules26164976