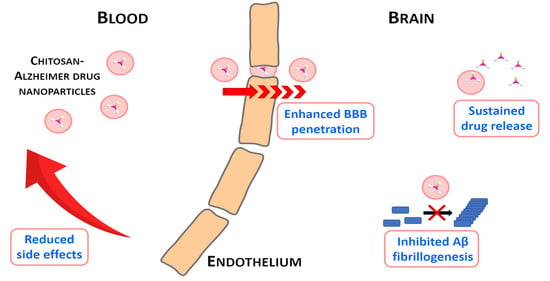
Use of Biodegradable, Chitosan-Based Nanoparticles in the Treatment of Alzheimer’s Disease
Abstract
:1. Introduction
- increased inflammatory response
- accumulation of reactive oxygen species (ROS)
- deposition of amyloid-beta (Aβ) protein
- acetylcholine (ACh) deficiency
- deposition of neurofibrillary tangles (NFTs) of tau proteins
- metal ion dynamic equilibrium disorder
2. Use of Chitosan Nanoparticles in the Treatment of Alzheimer’s Disease
2.1. Chitosan - Acetylcholinesterase Inhibitor Nanoparticles
2.2. Chitosan- Herbal Active Ingredient Nanoparticles
2.3. Chitosan NPs Combined with Other Therapeutic Agents
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Tamilselvan, N.; Raghavan, C.V. Formulation and characterization of anti-alzheimer’s drug loaded chitosan nanoparticles and its In vitro biological evaluation. J. Young Pharm. 2015, 7, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh, G.; Fallahi, Z.; Khanmohammadi, M.; Elmizadeh, H. Effect of Magnetic Tacrine-Loaded Chitosan Nanoparticles on Spatial Learning, Memory, Amyloid Precursor Protein and Seladin-1 Expression in the Hippocampus of Streptozotocin-Exposed Rats. Int. Clin. Neurosci. J. 2016, 3, 25–31. [Google Scholar] [CrossRef]
- Ouyang, Q.Q.; Zhao, S.; Li, S.D.; Song, C. Application of chitosan, chitooligosaccharide, and their derivatives in the treatment of Alzheimer’s disease. Mar. Drugs 2017, 15, 322. [Google Scholar] [CrossRef] [Green Version]
- Ferri, C.P.; Prince, M.; Brayne, C. Alzheimer’s Disease International. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Macauley, S.L.; Holtzman, D.M. Recent Advances from the Bench Toward the Bedside in Alzheimer’s Disease. EBioMedicine 2015, 2, 94–95. [Google Scholar] [CrossRef] [Green Version]
- Himmelstein, D.S.; Ward, S.M.; Lancia, J.K.; Patterson, K.R.; Binder, L.I. Tau as a therapeutic target in neurodegenerative disease. Pharmacol. Ther. 2012, 136, 8–22. [Google Scholar] [CrossRef] [Green Version]
- Oz, M.; Lorke, D.E.; Yang, K.-H.S.; Petroianu, G. On the interaction of β-amyloid peptides and α7-nicotinic acetylcholine receptors in Alzheimer’s disease. Curr. Alzheimer Res. 2013, 10, 618–630. [Google Scholar] [CrossRef]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef]
- Ghavami, A.; Hirst, W.D.; Novak, T.J. Selective phosphodiesterase (PDE)-4 inhibitors a novel approach to treating memory deficit? Drugs R D 2006, 7, 63–71. [Google Scholar] [CrossRef]
- Meinert, C.; McCaffrey, L.D.; Breitner, J.C.S. Alzheimer’s Disease Anti-Inflammatory Prevention Trial: Design, methods, and baseline results. Alzheimer’s Dement. 2009, 5, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.; Alonso, M.; Castro, A.; Pérez, C.; Moreno, F.J. First non-ATP competitive glycogen synthase kinase 3 β (GSK-3β) inhibitors: Thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J. Med. Chem. 2002, 45, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Wischik, C.M.; Edwards, P.C.; Lai, R.Y.K.; Roth, M.; Harrington, C.R. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl. Acad. Sci. USA 1996, 93, 11213–11218. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Banerjee, P.; Laferla, F.M.; Selkoe, D.J. Alzheimer’s Disease: Genes, Proteins, and Therapy. Physiol. Rev. 2010, 81, 741–766. [Google Scholar] [CrossRef]
- Sampson, E.L.; Jenagaratnam, L.; Mcshane, R. Metal protein attenuating compounds for the treatment of Alzheimer’s dementia. Cochrane Database Syst. Rev. 2014, 2014, CD005380. [Google Scholar] [CrossRef] [PubMed]
- Engelborghs, S.; Gilles, C.; Ivanoiu, A.; Vandewoude, M. Rationale and clinical data supporting nutritional intervention in Alzheimer’s disease. Acta Clin. Belg. 2014, 69, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, K.; Ikehara, S. Stem cell treatment for Alzheimer’s disease. Int. J. Mol. Sci. 2014, 15, 19226–19238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, S.S.; Shen, L.L.; Zhu, C.; Bu, X.L.; Liu, Y.H.; Liu, C.H.; Yao, X.Q.; Zhang, L.L.; Zhou, H.D.; Walker, D.G.; et al. Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer’s disease. Transl. Psychiatry 2016, 6. [Google Scholar] [CrossRef]
- Kaur, S.P.; Rao, R.; Hussain, A.; Khatkar, S. Preparation and characterization of rivastigmine loaded chitosan nanoparticles. J. Pharm. Sci. Res. 2011, 3, 1227–1232. [Google Scholar]
- Barbu, E.; Molnàr, É.; Tsibouklis, J.; Górecki, D.C. The potential for nanoparticle-based drug delivery to the brain: Overcoming the blood-brain barrier. Expert Opin. Drug Deliv. 2009, 6, 553–565. [Google Scholar] [CrossRef]
- Pardridge, W.M. Biopharmaceutical drug targeting to the brain. J. Drug Target. 2010, 18, 157–167. [Google Scholar] [CrossRef]
- Alam, M.I.; Beg, S.; Samad, A.; Baboota, S.; Kohli, K.; Ali, J.; Ahuja, A.; Akbar, M. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 2010, 40, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Cho, J.; Ko, Y.T. Investigation on the effect of nanoparticle size on the blood–brain tumour barrier permeability by in situ perfusion via internal carotid artery in mice. J. Drug Target. 2019, 27, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, S.; Janigro, D.; Hossain, M.; Oby, E.; Rapp, E.; Cucullo, L. Side by side comparison between dynamic versus static models of blood-brain barrier in vitro: A permeability study. Brain Res. 2006, 1109, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chigumira, W.; Maposa, P.; Gadaga, L.L.; Dube, A.; Tagwireyi, D.; Maponga, C.C. Preparation and Evaluation of Pralidoxime-Loaded PLGA Nanoparticles as Potential Carriers of the Drug across the Blood Brain Barrier. J. Nanomater. 2015, 2015, 692672. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Reimold, I.; Domke, D.; Bender, J.; Seyfried, C.A.; Radunz, H.E.; Fricker, G. Delivery of nanoparticles to the brain detected by fluorescence microscopy. Eur. J. Pharm. Biopharm. 2008, 70, 627–632. [Google Scholar] [CrossRef]
- Karthivashan, G.; Ganesan, P.; Park, S.Y.; Kim, J.S.; Choi, D.K. Therapeutic strategies and nano-drug delivery applications in management of ageing alzheimer’s disease. Drug Deliv. 2018, 25, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Mathew, G.E.; Uddin, M.S.; Kim, H.; Mathew, B. Advancements in nanotherapeutics for Alzheimer’s disease: Current perspectives. J. Pharm. Pharmacol. 2019, 71, 1370–1383. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.M.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood-brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef]
- Samadder, A.; Das, J.; Das, S.; De, A.; Saha, S.K.; Bhattacharyya, S.S.; Khuda-Bukhsh, A.R. Poly(lactic-co-glycolic) acid loaded nano-insulin has greater potentials of combating arsenic induced hyperglycemia in mice: Some novel findings. Toxicol. Appl. Pharmacol. 2013, 267, 57–73. [Google Scholar] [CrossRef]
- Hu, X.; Tulsieram, K.L.; Zhou, Q.; Mu, L.; Wen, J. Polymeric nanoparticle-aptamer bioconjugates can diminish the toxicity of mercury in vivo. Toxicol. Lett. 2012, 208, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Manek, E.; Tombácz, E.; Geissler, E.; László, K. Search for the origin of discrepancies in osmotic measurements of the PNIPAM - water system. Period. Polytech. Chem. Eng. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- László, K.; Manek, E.; Vavra, S.; Geissler, E.; Domján, A. Host guest interactions in poly(N-isopropylacrylamide) hydrogels. Chem. Lett. 2012, 41. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.; Samanta, M.K.; Muthu, M.S.; Vinothapooshan, G. Design and evaluation of chitosan nanoparticles as novel drug carrier for the delivery of rivastigmine to treat Alzheimer’s disease. Ther. Deliv. 2011, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Hassani, S.; Laouini, A.; Fessi, H.; Charcosset, C. Preparation of chitosan-TPP nanoparticles using microengineered membranes - Effect of parameters and encapsulation of tacrine. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 482, 34–43. [Google Scholar] [CrossRef]
- Patel, T.; Zhou, J.; Piepmeier, J.M.; Saltzman, W.M. Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 701–705. [Google Scholar] [CrossRef] [Green Version]
- Fazil, M.; Md, S.; Haque, S.; Kumar, M.; Baboota, S.; Sahni, J.K.; Ali, J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012, 47, 6–15. [Google Scholar] [CrossRef]
- Sarvaiya, J.; Agrawal, Y.K. Chitosan as a suitable nanocarrier material for anti-Alzheimer drug delivery. Int. J. Biol. Macromol. 2015, 72, 454–465. [Google Scholar] [CrossRef]
- Jaruszewski, K.M.; Ramakrishnan, S.; Poduslo, J.F.; Kandimalla, K.K. Chitosan enhances the stability and targeting of immuno-nanovehicles to cerebro-vascular deposits of Alzheimer’s disease amyloid protein. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Mehmood Khan, T.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Farid, R.M.; Elgamal, S.S. Complexation as an approach to entrap cationic drugs into cationic nanoparticles administered intranasally for Alzheimer’s disease management: Preparation and detection in rat brain. Drug Dev. Ind. Pharm. 2015, 41, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Tomaro-Duchesneau, C.; Prakash, S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials 2013, 34, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Optimization of brain targeted chitosan nanoparticles of Rivastigmine for improved efficacy and safety. Int. J. Biol. Macromol. 2013, 59, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.R.; Nazarians, L.A.; Giii, M.A. New Drugs A Review of Rivastigmine: Cholinesterase Inhibitor A Reversible. East 2003, 1634–1653. [Google Scholar]
- Kurz, A.; Farlow, M.; Lefèvre, G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer’s disease: A review. Int. J. Clin. Pract. 2009, 63, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Khemariya, R.P.; Khemariya, P.S. New-fangled approach in the management of Alzheimer by Formulation of Polysorbate 80 Coated Chitosan Nanoparticles of Rivastigmine for brain delivery and their in vivo evaluation. Int. J. Curr. Res. Med. Sci. 2016, 215, 18–29. [Google Scholar]
- Di Stefano, A.; Iannitelli, A.; Laserra, S.; Sozio, P. Drug delivery strategies for Alzheimer’s disease treatment. Expert Opin. Drug Deliv. 2011, 8, 581–603. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Haertel, C.; Maelicke, A.; Montag, D. Galantamine slows down plaque formation and behavioral decline in the 5XFAD mouse model of Alzheimer’s disease. PLoS ONE 2014, 9, 1–12. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Farid, R.M.; Helmy, M.W.; ElGamal, S.S. Pharmacological, toxicological and neuronal localization assessment of galantamine/chitosan complex nanoparticles in rats: Future potential contribution in Alzheimer’s disease management. Drug Deliv. 2016, 23, 3111–3122. [Google Scholar] [CrossRef]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Wagner, S.; Büchel, C.; Von Briesen, H.; Kreuter, J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J. Control. Release 2009, 137, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Reichman, W.E. Current pharmacologic options for patients with Alzheimer’s disease. Ann. Gen. Hosp. Psychiatry 2003, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Birks, J. Cholinesterase inhibitors for Alzheimer disease. JAMA 2003, 289. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kettle, C.; Morton, D.W. A molecular approach in drug development for Alzheimer’s disease. Biomed. Pharmacother. 2018, 106, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.J.; Zeng, X.S.; Song, X.Q.; Zhang, P.P.; Chen, L. Diabetes mellitus and Alzheimer’s disease: The protection of epigallocatechin-3-gallate in streptozotocin injection-induced models. Front. Pharmacol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.S.; Ramasamy, M.; Suresh, B. Chitosan nanoparticles as a new delivery system for the anti-Alzheimer drug tacrine. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 144–152. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Abbasi, S.H. Herbal medicine in the treatment of Alzhelmer’s disease. Am. J. Alzheimers Dis. Other Demen. 2006, 21, 113–118. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Shukla, P.K.; Khanna, V.K.; Khan, M.Y.; Srimal, R.C. Protective effect of curcumin against lead neurotoxicity in rat. Hum. Exp. Toxicol. 2003, 22, 653–658. [Google Scholar] [CrossRef]
- Reeta, K.; Mehla, J.; Gupta, Y. Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res. 2009, 1301, 52–60. [Google Scholar] [CrossRef]
- Shishodia, S.; Singh, T.; Chaturvedi, M.M. Modulation of transcription factors by curcumin. Adv. Exp. Med. Biol. 2007, 595, 127–148. [Google Scholar] [CrossRef]
- Wahlstrom, B.; Blennow, G. Study on Fate of Curcumin in Rat. Acta Pharmacol. Toxicol. 1978, 43, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, W.; Tungpradit, W.; Plianbangchang, P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int. J. Pharm. 2007, 337, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.; Maitra, A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnology 2007, 5, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Rabanel, J.M.; Faivre, J.; Paka, G.D.; Ramassamy, C.; Hildgen, P.; Banquy, X. Effect of polymer architecture on curcumin encapsulation and release from PEGylated polymer nanoparticles: Toward a drug delivery nano-platform to the CNS. Eur. J. Pharm. Biopharm. 2015, 96, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Zheng, Y.; Wang, Q.; Zhao, L. Curcumin-loaded chitosan–bovine serum albumin nanoparticles potentially enhanced Aβ 42 phagocytosis and modulated macrophage polarization in Alzheimer’s disease. Nanoscale Res. Lett. 2018, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.K.; Bansal, P.; Bhardwaj, R.K.; Velpandian, T. Comparative anti-nociceptive, anti-inflammatory and toxicity profile of nimesulide vs nimesulide and piperine combination. Pharmacol. Res. 2000, 41, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.H.; Fu, Q.H.; Liu, Y.; Jiang, K.; Guo, Q.M.; Chen, Q.Y.; Yan, B.; Wang, Q.Q.; Shen, J.G. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol. Sin. 2012, 33, 523–530. [Google Scholar] [CrossRef]
- Mishra, A.; Punia, J.K.; Bladen, C.; Zamponi, G.W.; Goel, R.K. Anticonvulsant mechanisms of piperine, a piperidine alkaloid. Channels 2015, 9, 317–323. [Google Scholar] [CrossRef]
- Lee, S.A.; Hong, S.S.; Han, X.H.; Hwang, J.S.; Oh, G.J.; Lee, K.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Piperine from the fruits of Piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chem. Pharm. Bull. 2005, 53, 832–835. [Google Scholar] [CrossRef] [Green Version]
- McNamara, F.N.; Randall, A.; Gunthorpe, M.J. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1). Br. J. Pharmacol. 2005, 144, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Yin, Y.; Vu, S.; Yang, F.; Yarov-Yarovoy, V.; Tian, Y.; Zheng, J. A distinct structural mechanism underlies TRPV1 activation by piperine. Biochem. Biophys. Res. Commun. 2019, 516, 365–372. [Google Scholar] [CrossRef]
- Priprem, A.; Sutthiparinyanont, S.; Wattanathorn, J. Antidepressant and cognitive activities of intranasal piperine-encapsulated liposomes. Adv. Biosci. Biotechnol. 2011, 2, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Sastre, J.; Mosges, R. Local and systemic safety of intranasal corticosteroids. J. Investig. Allergol. Clin. Immunol. 2012, 22, 1–12. [Google Scholar]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal Piperine-Loaded Chitosan Nanoparticles as Brain-Targeted Therapy in Alzheimer’s Disease: Optimization, Biological Efficacy, and Potential Toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef]
- Alam, S.; Khan, Z.I.; Mustafa, G.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: A pharmacoscintigraphic study. Int. J. Nanomed. 2012, 7, 5705–5718. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M.A.; Nagi, M.N.; El-Khatib, A.S.; Al-Bekairi, A.M. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and dt-diaphorase in different tissues of mice: A possible mechanism of action. Cell Biochem. Funct. 2002, 20, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Al-Omar, F.A.; Nagi, M.N. Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur. J. Pharmacol. 2006, 543, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, M.S. Anti-inflammatory, Antipyretic, and Analgesic Agents of Nigella sativa. J. Ethnopharmacol. 2001, 76, 45–48. [Google Scholar] [CrossRef]
- Baell, J.B. Feeling Nature’s PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, J.; Irwin, R.W.; Gallaher, T.K.; Brinton, R.D. Estradiol in vivo regulation of brain mitochondrial proteome. J. Neurosci. 2007, 27, 14069–14077. [Google Scholar] [CrossRef] [PubMed]
- Schupf, N.; Winsten, S.; Patel, B.; Pang, D.; Ferin, M.; Zigman, W.B.; Silverman, W.; Mayeux, R. Bioavailable estradiol and age at onset of Alzheimer’s disease in postmenopausal women with Down syndrome. Neurosci. Lett. 2006, 406, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Kölsch, H.; Rao, M.L. Neuroprotective effects of estradiol-17β: Implications for psychiatric disorders. Arch. Womens Ment. Health 2002, 5, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chi, N.; Tang, X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur. J. Pharm. Biopharm. 2008, 70, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Ugen, K.E.; Dickey, C.; Hardyk, J.; Duff, K.; Jantzen, P.; Dicarlo, G.; Wilcock, D.; Connor, K.; Hatcher, J.; Hope, C.; et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature 2000, 408, 982–985. [Google Scholar] [CrossRef]
- Lopez, O.L.; Rabin, B.S.; Huff, F.J.; Rezek, D.; Reinmuth, O.M. Serum autoantibodies in patients with alzheimer’s disease and vascular dementia and in nondemented control subjects. Stroke 1992, 23, 1078–1083. [Google Scholar] [CrossRef] [Green Version]
- Soto, C. Plaque busters: Strategies to inhibit amyloid formation in Alzheimer’s disease. Mol. Med. Today 1999, 5, 343–350. [Google Scholar] [CrossRef]
- Fradinger, E.A.; Monien, B.H.; Urbanc, B.; Lomakin, A.; Tan, M.; Li, H.; Spring, S.M.; Condron, M.M.; Cruz, L.; Xie, C.W.; et al. C-terminal peptides coassemble into Aβ42 oligomers and protect neurons against Aβ42-induced neurotoxicity. Proc. Natl. Acad. Sci. USA 2008, 105, 14175–14180. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wu, L. Amyloid-beta associated with chitosan nano-carrier has favorable immunogenicity and permeates the BBB. AAPS PharmSciTech 2009, 10, 900–905. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Dong, X.; Sun, Y. Charge effects of self-assembled chitosan-hyaluronic acid nanoparticles on inhibiting amyloid β-protein aggregation. Carbohydr. Res. 2018, 461, 11–18. [Google Scholar] [CrossRef]
- Yang, L.; Gao, S.; Asghar, S.; Liu, G.; Song, J.; Wang, X.; Ping, Q.; Zhang, C.; Xiao, Y. Hyaluronic acid/chitosan nanoparticles for delivery of curcuminoid and its in vitro evaluation in glioma cells. Int. J. Biol. Macromol. 2015, 72, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Lallana, E.; Rios De La Rosa, J.M.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/Hyaluronic Acid Nanoparticles: Rational Design Revisited for RNA Delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Misiak, H.; Misiak, H. Methylene Blue. The long and winding road from satin to brain: Part 1. J. Psychosoc. Nurs. 2016, 54, 21–24. [Google Scholar] [CrossRef]
- Wainwright, M.; Crossley, K.B. Methylene Blue—A therapeutic dye for all seasons? J. Chemother. 2002, 14, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and Molecular Actions of Methylene Blue in the Nervous System. Med. Res. Rev. 2010, 31, 93–117. [Google Scholar] [CrossRef] [Green Version]
- De Castro, A.A.; Soares, F.V.; Pereira, A.F.; Polisel, D.A.; Caetano, M.S.; Leal, D.H.S.; Da Cunha, E.F.F.; Nepovimova, E.; Kuca, K.; Ramalho, T.C. Non-conventional compounds with potential therapeutic effects against Alzheimer’s disease. Expert Rev. Neurother. 2019, 19, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Petroianu, G.A. Methylene blue and Alzheimer’s disease. Biochem. Pharmacol. 2009, 78, 927–932. [Google Scholar] [CrossRef]
- Al Mansouri, A.S.; Lorke, D.E.; Nurulain, S.M.; Ashoor, A.; Yang Keun-Hang, S.; Petroianu, G.; Isaev, D.; Oz, M. Methylene Blue Inhibits the Function of α7-Nicotinic Acetylcholine Receptors. CNS Neurol. Disord. Drug Targets 2012, 11, 791–800. [Google Scholar] [CrossRef]
- Callaway, N.L.; Riha, P.D.; Bruchey, A.K.; Munshi, Z.; Gonzalez-Lima, F. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol. Biochem. Behav. 2004, 77, 175–181. [Google Scholar] [CrossRef]
- Deiana, S.; Harrington, C.R.; Wischik, C.M.; Riedel, G. Methylthioninium chloride reverses cognitive deficits induced by scopolamine: Comparison with rivastigmine. Psychopharmacology 2009, 202, 53–65. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.L.; Petty, J.; Harris, A.B.; Inukai, J. Supravital staining of mammalian brain with intraarterial methylene blue followed by pressurized oxygen. Stain Technol. 1968, 43, 197–201. [Google Scholar] [CrossRef]
- Peter, C.; Hongwan, D.; Küpfer, A.; Lauterburg, B.H. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 2000, 56, 247–250. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Qu, Z.C.; Cobb, C.E. Reduction and uptake of methylene blue by human erythrocytes. Am. J. Physiol. Cell Physiol. 2004, 286, 1390–1399. [Google Scholar] [CrossRef] [Green Version]
- Rengelshausen, J.; Burhenne, J.; Fröhlich, M.; Tayrouz, Y.; Singh, S.K.; Riedel, K.D.; Müller, O.; Hoppe-Tichy, T.; Haefeli, W.E.; Mikus, G.; et al. Pharmacokinetic interaction of chloroquine and methylene blue combination against malaria. Eur. J. Clin. Pharmacol. 2004, 60, 709–715. [Google Scholar] [CrossRef]
- Buchholz, K.; Schirmer, R.H.; Eubel, J.K.; Akoachere, M.B.; Dandekar, T.; Becker, K.; Gromer, S. Interactions of methylene blue with human disulfide reductases and their orthologues from Plasmodium falciparum. Antimicrob. Agents Chemother. 2008, 52, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Palma-Chavez, J.; Kim, W.; Serafino, M.; Jo, J.; Charoenphol, P.; Applegate, B. Methylene blue-filled biodegradable polymer particles as a contrast agent for optical coherence tomography. Biomed. Opt. Express 2020, 11, 4255. [Google Scholar] [CrossRef]
- Lee, B.I., II; Suh, Y.S.; Chung, Y.J.; Yu, K.; Park, C.B. Shedding Light on Alzheimer’s β-Amyloidosis: Photosensitized Methylene Blue Inhibits Self-Assembly of β-Amyloid Peptides and Disintegrates Their Aggregates. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hashweh, N.N.; Bartochowski, Z.; Khoury, R.; Grossberg, G.T. An evaluation of hydromethylthionine as a treatment option for Alzheimer’s disease. Expert Opin. Pharmacother. 2020, 21, 619–627. [Google Scholar] [CrossRef]
- Cannavà, C.; Stancanelli, R.; Marabeti, M.R.; Venuti, V.; Cascio, C.; Guarneri, P.; Bongiorno, C.; Sortino, G.; Majolino, D.; Mazzaglia, A.; et al. Nanospheres based on PLGA/amphiphilic cyclodextrin assemblies as potential enhancers of Methylene Blue neuroprotective effect. RSC Adv. 2016, 6, 16720–16729. [Google Scholar] [CrossRef]
- Alejandra Gutiérrez-Valenzuela, C.; Rodríguez-Córdova, R.; Hernández-Giottonini, Y.; Guerrero-Germán, P.; Lucero-Acuña, A. Methylene Blue Loaded PLGA Nanoparticles: Combined Emulsion, Drug Release Analysis and Photodynamic Activity. Microsc. Microanal. 2017, 23, 1212–1213. [Google Scholar] [CrossRef] [Green Version]
- Jinwal, K.U.; Groshev, A.; Zhang, J.; Grover, A.; Sutariya, B. Preparation and Characterization of Methylene blue Nanoparticles for Alzheimer’s Disease and Other Tauopathies. Curr. Drug Deliv. 2014, 11, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Gill, J.M.; Ranjan, A.P.; Vishwanatha, J.K. Development and Characterization of Methylene Blue Oleate Salt-Loaded Polymeric Nanoparticles and their Potential Application as a Treatment for Glioblastoma. J. Nanomed. Nanotechnol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, A.; Hirani, A.; Sutariya, V. Blood-Brain Barrier Permeation of Paclitaxel. Nirma Univ. J. Pharm. Sci. 2014, 1, 71–78. [Google Scholar]
- Jyothi, M.S.; Angadi, V.J.; Kanakalakshmi, T.V.; Padaki, M.; Geetha, B.R.; Soontarapa, K. Magnetic Nanoparticles Impregnated, Cross-Linked, Porous Chitosan Microspheres for Efficient Adsorption of Methylene Blue from Pharmaceutical Waste Water. J. Polym. Environ. 2019, 27, 2408–2418. [Google Scholar] [CrossRef]
- Ramezani, S.; Zahedi, P.; Bahrami, S.H.; Nemati, Y. Microfluidic Fabrication of Nanoparticles Based on Ethyl Acrylate-Functionalized Chitosan for Adsorption of Methylene Blue from Aqueous Solutions. J. Polym. Environ. 2019, 27, 1653–1665. [Google Scholar] [CrossRef]
- Deng, W.; Tang, S.; Zhou, X.; Liu, Y.; Liu, S.; Luo, J. Honeycomb-like structure-tunable chitosan-based porous carbon microspheres for methylene blue efficient removal. Carbohydr. Polym. 2020, 247, 116736. [Google Scholar] [CrossRef]
- Hosseini, F.; Sadighian, S.; Hosseini-Monfared, H.; Mahmoodi, N.M. Dye removal and kinetics of adsorption by magnetic chitosan nanoparticles. Desalin. Water Treat. 2016, 57, 24378–24386. [Google Scholar] [CrossRef]
- Khdair, A.; Hamad, I.; Alkhatib, H.; Bustanji, Y.; Mohammad, M.; Tayem, R.; Aiedeh, K. Modified-chitosan nanoparticles: Novel drug delivery systems improve oral bioavailability of doxorubicin. Eur. J. Pharm. Sci. 2016, 93, 38–44. [Google Scholar] [CrossRef]
- Tronci, G.; Ajiro, H.; Russell, S.J.; Wood, D.J.; Akashi, M. Tunable drug-loading capability of chitosan hydrogels with varied network architectures. Acta Biomater. 2014, 10, 821–830. [Google Scholar] [CrossRef]
- Koc, F.E.; Altıncekic, T.G. Investigation of gelatin/chitosan as potential biodegradable polymer films on swelling behavior and methylene blue release kinetics. Polym. Bull. 2020. [Google Scholar] [CrossRef]
- Şenel, M.; Ebru Koç, F. Controlled release of methylene blue from layer-by-layer assembled chitosan/polyacrylic acid. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 258–262. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Chenite, A.; Chaput, C.; Guirguis, S.; Leroux, J.C. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int. J. Pharm. 2000, 203, 89–98. [Google Scholar] [CrossRef]
- De, S.; Robinson, D. Polymer relationships during preparation of chitosan-alginate and poly-l-lysine-alginate nanospheres. J. Control. Release 2003, 89, 101–112. [Google Scholar] [CrossRef]
- Aghabegi Moghanjoughi, A.; Khoshnevis, D.; Zarrabi, A. A concise review on smart polymers for controlled drug release. Drug Deliv. Transl. Res. 2016, 6, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, A.; Sasaki, D.; Shiohara, A.; Iwatsubo, T.; Tomita, T.; Sohma, Y.; Kanai, M. Attenuation of the aggregation and neurotoxicity of amyloid-b peptides by catalytic photooxygenation. Angew. Chem. Int. Ed. 2014, 53, 1382–1385. [Google Scholar] [CrossRef]
- Lee, B.I., II; Lee, S.; Suh, Y.S.; Lee, J.S.; Kim, A.; Kwon, O.-Y.; Yu, K.; Park, C.B. Photoexcited Porphyrins as a Strong Suppressor of β-Amyloid Aggregation and Synaptic Toxicity. Angew. Chemie 2015, 127, 11634–11638. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Bernegossi, J.; De Freitas, L.M.; Fontana, C.R.; Chorilli, M.; Grumezescu, A.M. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: A review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Tang, W.; Xu, H.; Park, E.J.; Philbert, M.A.; Kopelman, R. Encapsulation of methylene blue in polyacrylamide nanoparticle platforms protects its photodynamic effectiveness. Biochem. Biophys. Res. Commun. 2008, 369, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Disanto, A.R.; Wagner, J.G. Pharmacokinetics of highly ionized drugs II: Methylene blue—absorption, metabolism, and excretion in man and dog after oral administration. J. Pharm. Sci. 1972, 61, 1086–1090. [Google Scholar] [CrossRef]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.M.F.; De Annunzio, S.R.; Victorelli, F.D.; Frade, M.L.; Ferreira, P.S.; Chorilli, M.; Fontana, C.R. Chitosan-Based Drug Delivery Systems for Optimization of Photodynamic Therapy: A Review. AAPS PharmSciTech 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Darabpour, E.; Kashef, N.; Mashayekhan, S. Chitosan nanoparticles enhance the efficiency of methylene blue-mediated antimicrobial photodynamic inactivation of bacterial biofilms: An in vitro study. Photodiagnosis Photodyn. Ther. 2016, 14, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Koo, H.; Jeong, H.; Huh, M.S.; Choi, Y.; Jeong, S.Y.; Byun, Y.; Choi, K.; Kim, K.; Kwon, I.C. Comparative study of photosensitizer loaded and conjugated glycol chitosan nanoparticles for cancer therapy. J. Control. Release 2011, 152, 21–29. [Google Scholar] [CrossRef]
- Tsai, W.H.; Yu, K.H.; Huang, Y.C.; Lee, C.I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef] [Green Version]
- Chien, H.F.; Chen, C.P.; Chen, Y.C.; Chang, P.H.; Tsai, T.; Chen, C.T. The use of chitosan to enhance photodynamic inactivation against Candida albicans and its drug-resistant clinical isolates. Int. J. Mol. Sci. 2013, 14, 7445–7456. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.P.; Chen, C.T.; Tsai, T. Chitosan nanoparticles for antimicrobial photodynamic inactivation: Characterization and in vitro investigation. Photochem. Photobiol. 2012, 88, 570–576. [Google Scholar] [CrossRef]
- Frade, M.L.; De Annunzio, S.R.; Calixto, G.M.F.; Victorelli, F.D.; Chorilli, M.; Fontana, C.R. Assessment of chitosan-based hydrogel and photodynamic inactivation against propionibacterium acnes. Molecules 2018, 23, 473. [Google Scholar] [CrossRef] [Green Version]
- Xuan Du, D.; Xuan Vuong, B.; Mai, H.D. Study on Preparation of Water-Soluble Chitosan with Varying Molecular Weights and Its Antioxidant Activity. Adv. Mater. Sci. Eng. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.C.; Hon, M.H. The effect of the molecular weight of chitosan nanoparticles and its application on drug delivery. Microchem. J. 2009, 92, 87–91. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Whu, S.W.; Tsai, C.L.; Wu, Y.H.; Chen, H.W.; Hsieh, K.H. Chitosan as scaffold materials: Effects of molecular weight and degree of deacetylation. J. Polym. Res. 2004, 11, 141–147. [Google Scholar] [CrossRef]
- Zhang, H.; Neau, S.H. In vitro degradation of chitosan by a commercial enzyme preparation: Effect of molecular weight and degree of deacetylation. Biomaterials 2001, 22, 1653–1658. [Google Scholar] [CrossRef]
- Chang, S.H.; Wu, C.H.; Tsai, G.J. Effects of chitosan molecular weight on its antioxidant and antimutagenic properties. Carbohydr. Polym. 2018, 181, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Nanoparticle mediated brain targeted delivery of gallic acid: In vivo behavioral and biochemical studies for improved antioxidant and antidepressant-like activity. Drug Deliv. 2012, 19, 378–391. [Google Scholar] [CrossRef]
- Pereverzeva, E.; Treschalin, I.; Bodyagin, D.; Maksimenko, O.; Langer, K.; Dreis, S.; Asmussen, B.; Kreuter, J.; Gelperina, S. Influence of the formulation on the tolerance profile of nanoparticle-bound doxorubicin in healthy rats: Focus on cardio- and testicular toxicity. Int. J. Pharm. 2007, 337, 346–356. [Google Scholar] [CrossRef]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood-brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef]
- Wagner, S.; Zensi, A.; Wien, S.L.; Tschickardt, S.E.; Maier, W.; Vogel, T.; Worek, F.; Pietrzik, C.U.; Kreuter, J.; Von Briesen, H. Uptake mechanism of ApoE-modified nanoparticles on brain capillary endothelial cells as a blood-brain barrier model. PLoS ONE 2012, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chonpathompikunlert, P.; Wattanathorn, J.; Muchimapura, S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem. Toxicol. 2010, 48, 798–802. [Google Scholar] [CrossRef]
- Kreuter, J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. J. Nanosci. Nanotechnol. 2004, 4, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Tamai, I. Blood-brain barrier function of P-glycoprotein. Adv. Drug Deliv. Rev. 1997, 25, 287–298. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems - A review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Khan, M.K.; Nigavekar, S.S.; Minc, L.D.; Kariapper, M.S.T.; Nair, B.M.; Lesniak, W.G.; Balogh, L.P. In vivo biodistribution of dendrimers and dendrimer nanocomposites - Implications for cancer imaging and therapy. Technol. Cancer Res. Treat. 2005, 4, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef]
- Liu, H.; Ojha, B.; Morris, C.; Jiang, M.; Wojcikiewicz, E.P.; Rao, P.P.N.; Du, D. Positively Charged Chitosan and N-Trimethyl Chitosan Inhibit Aβ40 Fibrillogenesis. Biomacromolecules 2015, 16, 2363–2373. [Google Scholar] [CrossRef]
- Assarsson, A.; Hellstrand, E.; Cabaleiro-Lago, C.; Linse, S. Charge dependent retardation of amyloid β aggregation by hydrophilic proteins. ACS Chem. Neurosci. 2014, 5, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Cabaleiro-Lago, C.; Quinlan-Pluck, F.; Lynch, I.; Dawson, K.A.; Linse, S. Dual effect of amino modified polystyrene nanoparticles on amyloid β protein fibrillation. ACS Chem. Neurosci. 2010, 1, 279–287. [Google Scholar] [CrossRef]
- Luo, J.; Yu, C.H.; Yu, H.; Borstnar, R.; Kamerlin, S.C.L.; Gräslund, A.; Abrahams, J.P.; Wärmländer, S.K.T.S. Cellular polyamines promote amyloid-Beta (Aβ) peptide fibrillation and modulate the aggregation pathways. ACS Chem. Neurosci. 2013, 4, 454–462. [Google Scholar] [CrossRef]
- Assarsson, A.; Linse, S.; Cabaleiro-Lago, C. Effects of polyamino acids and polyelectrolytes on amyloid β fibril formation. Langmuir 2014, 30, 8812–8818. [Google Scholar] [CrossRef]
- Chan, H.M.; Xiao, L.; Yeung, K.M.; Ho, S.L.; Zhao, D.; Chan, W.H.; Li, H.W. Effect of surface-functionalized nanoparticles on the elongation phase of beta-amyloid (1-40) fibrillogenesis. Biomaterials 2012, 33, 4443–4450. [Google Scholar] [CrossRef]
- Xu, Y.; Takai, M.; Ishihara, K. Protein adsorption and cell adhesion on cationic, neutral, and anionic 2-methacryloyloxyethyl phosphorylcholine copolymer surfaces. Biomaterials 2009, 30, 4930–4938. [Google Scholar] [CrossRef] [PubMed]
- Elbassal, E.A.; Liu, H.; Morris, C.; Wojcikiewicz, E.P.; Du, D. Effects of Charged Cholesterol Derivatives on Aβ40 Amyloid Formation. J. Phys. Chem. B 2016, 120, 59–68. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.H.; Lee, H.; Nam, J.M. How Do the Size, Charge and Shape of Nanoparticles Affect Amyloid β Aggregation on Brain Lipid Bilayer? Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Bag, S.; Chaudhury, S.; Pramanik, D.; DasGupta, S.; Dasgupta, S. Hydrophobic tail length plays a pivotal role in amyloid beta (25–35) fibril–surfactant interactions. Proteins Struct. Funct. Bioinforma. 2016, 84, 1213–1223. [Google Scholar] [CrossRef]
- Otzen, D.; Nielsen, P.H. We find them here, we find them there: Functional bacterial amyloid. Cell. Mol. Life Sci. 2008, 65, 910–927. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surfaces B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Manek, E.; Domján, A.; Menyhárd, A.; László, K. Host-guest interactions in poly(N-isopropylacrylamide) gel: A thermoanalytical approach. J. Therm. Anal. Calorim. 2015, 120. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Burgess, D.J. Effect of acidic pH on PLGA microsphere degradation and release. J. Control. Release 2007, 122, 338–344. [Google Scholar] [CrossRef]
- Domján, A.; Manek, E.; Geissler, E.; László, K. Host-guest interactions in poly(N-isopropylacrylamide) hydrogel seen by one- and two-dimensional 1H CRAMPS solid-state NMR spectroscopy. Macromolecules 2013, 46, 3118–3124. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Naki, T. Chitosan-based nanocarriers for nose to brain delivery. Appl. Sci. 2019, 9, 2219. [Google Scholar] [CrossRef] [Green Version]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Filley, C.; Halliday, W.; Kleinschmidt-DeMasters, B.K. The effects of toluene on the central nervous system. J. Am. Med. Assoc. 2004, 63, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Takigawa, T.; Endo, Y. Effects of glutaraldehyde exposure on human health. J. Occup. Health 2006, 48, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Tosi, G.; Costantino, L.; Ruozi, B.; Forni, F.; Vandelli, M.A. Polymeric nanoparticles for drug delivery to the central nervous system. Expert Opin. Drug Deliv. 2008, 5, 155–174. [Google Scholar] [CrossRef]
- Illum, L. Review Article Is nose-to-brain transport of drugs in man a reality? J. Pharm. Pharmacol. 2004, 56, 3–17. [Google Scholar] [CrossRef]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A. A New Horizon in Modifications of Chitosan: Syntheses and Applications. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 91–181. [Google Scholar] [CrossRef]
- Borchard, G.; Lueßen, H.L.; De Boer, A.G.; Verhoef, J.C.; Lehr, C.M.; Junginger, H.E. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: Effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J. Control. Release 1996, 39, 131–138. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005, 28, 202–208. [Google Scholar] [CrossRef]
- Hombach, J.; Bernkop-Schnürch, A. Chitosan solutions and particles: Evaluation of their permeation enhancing potential on MDCK cells used as blood brain barrier model. Int. J. Pharm. 2009, 376, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Neuroprotective properties of chitosan and its derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Khodagholi, F.; Eftekharzadeh, B.; Maghsoudi, N.; Rezaei, P.F. Chitosan prevents oxidative stress-induced amyloid β formation and cytotoxicity in NT2 neurons: Involvement of transcription factors Nrf2 and NF-κB. Mol. Cell. Biochem. 2010, 337, 39–51. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Hwang, J.W.; Han, Y.K.; Lee, J.S.; Kim, S.K.; Jeon, Y.J.; Moon, S.H.; Jeon, B.T.; Bahk, Y.Y.; et al. Sulfated chitosan oligosaccharides suppress LPS-induced NO production via JNK and NF-κB inactivation. Molecules 2014, 19, 18232–18247. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Sung, M.J.; Seo, S.B.; Yoo, S.J.; Lim, W.K.; Kim, H.M. Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid β peptide and interleukin-1β. Neurosci. Lett. 2002, 321, 105–109. [Google Scholar] [CrossRef]
- Je, J.Y.; Park, P.J.; Kim, S.K. Free radical scavenging properties of hetero-chitooligosaccharides using an ESR spectroscopy. Food Chem. Toxicol. 2004, 42, 381–387. [Google Scholar] [CrossRef]
- Byun, H.G.; Kim, Y.T.; Park, P.J.; Lin, X.; Kim, S.K. Chitooligosaccharides as a novel β-secretase inhibitor. Carbohydr. Polym. 2005, 61, 198–202. [Google Scholar] [CrossRef]
- Dai, X.; Hou, W.; Sun, Y.; Gao, Z.; Zhu, S.; Jiang, Z. Chitosan oligosaccharides inhibit/disaggregate fibrils and attenuate amyloid β-mediated neurotoxicity. Int. J. Mol. Sci. 2015, 16, 10526–10536. [Google Scholar] [CrossRef]
- Dai, X.; Chang, P.; Zhu, Q.; Liu, W.; Sun, Y.; Zhu, S.; Jiang, Z. Chitosan oligosaccharides protect rat primary hippocampal neurons from oligomeric β-amyloid 1-42-induced neurotoxicity. Neurosci. Lett. 2013, 554, 64–69. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Ngo, D.N.; Kim, S.K. Acetylcholinesterase inhibitory activity of novel chitooligosaccharide derivatives. Carbohydr. Polym. 2009, 78, 869–872. [Google Scholar] [CrossRef]
- Xu, W.; Huang, H.C.; Lin, C.J.; Jiang, Z.F. Chitooligosaccharides protect rat cortical neurons against copper induced damage by attenuating intracellular level of reactive oxygen species. Bioorganic Med. Chem. Lett. 2010, 20, 3084–3088. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhuge, X.; Yang, Y.; Gu, X.; Ding, F. The promotion of peripheral nerve regeneration by chitooligosaccharides in the rat nerve crush injury model. Neurosci. Lett. 2009, 454, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yang, Y.; Gu, X.; Ding, F. Chitooligosaccharides protect cultured hippocampal neurons against glutamate-induced neurotoxicity. Neurosci. Lett. 2008, 444, 270–274. [Google Scholar] [CrossRef] [PubMed]












| API | Additive | NP Synthesis Method | NP Size (nm) | NP Morphology | Encapsulation Efficacy * | References |
|---|---|---|---|---|---|---|
| rivastigmine | - | ionotropic gelation | 154 | spherical | 96% | Nagpal et al. [44] |
| - | ionotropic gelation | 44 | subspherical | 85% | Khemariya et al. [47] | |
| - | ionotropic gelation | 184 | spherical to subspherical | 86% | Fazil et al. [38] | |
| - | spontaneous emulsification | 47 | not specified | 74% | Wilson et al. [34] | |
| galantamine | - | ionotropic gelation | 182–190 | spherical to subspherical | 23% | Hanafy et al. [42,50] |
| tacrine | - | not specified | not specified | not specified | not specified | Hassanzadeh et al. [2] |
| - | ionotropic gelation | 50 | sub-spherical | 42–72% | Tamilselvan et al. [1] | |
| - | spontaneous emulsification | 41 | spherical | 80% | Wilson et al. [56] | |
| - | ionotropic gelation | 90–100 | not specified | 65% | Hassani et al. [36] | |
| curcumin | BSA | electrostatic interaction-based conjugation | 145 | spherical | 95% | Yang et al. [66] |
| piperine | - | ionotropic gelation | 249 | spherical | 82% | Elnaggar et al. [75] |
| thymoquinone | - | ionotropic gelation | 172 | spherical with smooth surface | 63% | Alam et al. [76] |
| 17β-estradiol | - | ionotropic gelation | 270 | not specified | 65% | Wang et al. [84] |
| Aβ subfragments | - | combined emulsification and crosslinking | 15 | spherical | 79% | Songjiang et al. [89] |
| no cargo | HA | electrostatic interaction induced self-assembly | 67–698 | not specified | - | Jiang et al. [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manek, E.; Darvas, F.; Petroianu, G.A. Use of Biodegradable, Chitosan-Based Nanoparticles in the Treatment of Alzheimer’s Disease. Molecules 2020, 25, 4866. https://doi.org/10.3390/molecules25204866
Manek E, Darvas F, Petroianu GA. Use of Biodegradable, Chitosan-Based Nanoparticles in the Treatment of Alzheimer’s Disease. Molecules. 2020; 25(20):4866. https://doi.org/10.3390/molecules25204866
Chicago/Turabian StyleManek, Eniko, Ferenc Darvas, and Georg A. Petroianu. 2020. "Use of Biodegradable, Chitosan-Based Nanoparticles in the Treatment of Alzheimer’s Disease" Molecules 25, no. 20: 4866. https://doi.org/10.3390/molecules25204866