Structural Evolution and Magnetic Properties of Gd2Hf2O7 Nanocrystals: Computational and Experimental Investigations
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis Procedure
2.2. Sample Characterization
2.3. Computational Methodology
3. Results and Discussion
3.1. Modeling of the OP-GHO Structures
3.2. Structural Parameters for the OP-GHO
3.3. X-Ray Diffraction and SEM Analysis
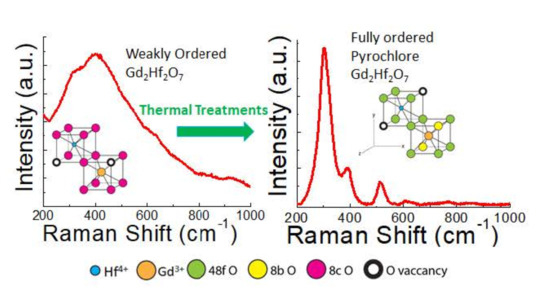
3.4. Raman Spectroscopy
3.5. Magnetic Properties
4. DFT Calculations in OP-GHO
4.1. Densities of States (DOS) Calculations
4.2. Spin Density Calculations
5. Overlap Populations COOP and COHP
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Avsar, A.; Ciarrocchi, A.; Pizzochero, M.; Unuchek, D.; Yazyev, O.V.; Kis, A. Defect induced, layer-modulated magnetism in ultrathin metallic PtSe2. Nat. Nanotechnol. 2019, 14, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Puretzky, A.A.; Yoshimura, A.; Sang, X.; Cui, Q.; Li, Y.; Liang, L.; Ghosh, A.W.; Zhao, H. Isotope-Engineering the Thermal Conductivity of Two-Dimensional MoS2. ACS Nano 2019, 13, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Medina, J.; Wang, Z.; Cruz-Silva, R.; Morelos-Gomez, A.; Wang, F.; Yao, X.; Terrones, M.; Endo, M. Defect Engineering and Surface Functionalization of Nanocarbons for Metal-Free Catalysis. Adv. Mater. 2019, 31, 1805717. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Wu, Q.; Cao, F.; Yang, D.; Shang, Y.; Ning, Z.; Zhang, W.; Zheng, W.; Yan, Y. Trifluoroacetate induced small-grained CsPbBr3 perovskite films result in efficient and stable light-emitting devices. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Castro Neto, A.; Guinea, F.; Peres, N.; Novoselov, K.; Geim, A. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, P.; Chung, C.; McMillan, J.; Tsai, M.; Lu, M.; Panoiu, N.; Wong, C.W. Photon transport enhanced by transverse Anderson localization in disordered superlattices. Nat. Phys. 2015, 11, 268. [Google Scholar] [CrossRef]
- Shamblin, J.; Feygenson, M.; Neuefeind, J.; Tracy, C.L.; Zhang, F.; Finkeldei, S.; Bosbach, D.; Zhou, H.; Ewing, R.C.; Lang, M. Probing disorder in isometric pyrochlore and related complex oxides. Nat. Mater. 2016, 15, 507. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.-I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414. [Google Scholar] [CrossRef]
- Durand, A.M.; Klavins, P.; Corruccini, L. Heat capacity of the frustrated magnetic pyrochlores Gd2Zr2O7 and Gd2Hf2O7. J. Phys. Condens. Matter 2008, 20, 235208. [Google Scholar] [CrossRef]
- Pokhrel, M.; Wahid, K.; Mao, Y. Systematic Studies on RE2Hf2O7:5%Eu3+ (RE = Y, La, Pr, Gd, Er, and Lu) Nanoparticles: Effects of the A-Site RE3+ Cation and Calcination on Structure and Photoluminescence. J. Phys. Chem. C 2016, 120, 14828–14839. [Google Scholar] [CrossRef]
- Pokhrel, M.; Alcoutlabi, M.; Mao, Y. Optical and X-ray induced luminescence from Eu3+ doped La2Zr2O7 nanoparticles. J. Alloy. Compd. 2017, 693, 719–729. [Google Scholar] [CrossRef] [Green Version]
- Sickafus, K.; Minervini, L.; Grimes, R.; Valdez, J.; Ishimaru, M.; Li, F.; McClellan, K.; Hartmann, T. Radiation tolerance of complex oxides. Science 2000, 289, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Ewing, R.C.; Weber, W.J.; Lian, J. Nuclear waste disposal—Pyrochlore (A2B2O7): Nuclear waste form for the immobilization of plutonium and “minor” actinides. J. Appl. Phys. 2004, 95, 5949–5971. [Google Scholar] [CrossRef] [Green Version]
- Sickafus, K.E.; Grimes, R.W.; Valdez, J.A.; Cleave, A.; Tang, M.; Ishimaru, M.; Corish, S.M.; Stanek, C.R.; Uberuaga, B.P. Radiation-induced amorphization resistance and radiation tolerance in structurally related oxides. Nat. Mater. 2007, 6, 217. [Google Scholar] [CrossRef] [PubMed]
- Wuensch, B.J.; Eberman, K.W.; Heremans, C.; Ku, E.M.; Onnerud, P.; Yeo, E.M.; Haile, S.M.; Stalick, J.K.; Jorgensen, J.D. Connection between oxygen-ion conductivity of pyrochlore fuel-cell materials and structural change with composition and temperature. Solid State Ion. 2000, 129, 111–133. [Google Scholar] [CrossRef]
- Abdou, M.; Gupta, S.K.; Zuniga, J.P.; Mao, Y. On structure and phase transformation of uranium doped La2Hf2O7 nanoparticles as an efficient nuclear waste host. Mater. Chem. Front. 2018, 2, 2201–2211. [Google Scholar] [CrossRef]
- Saha, S.; Prusty, S.; Singh, S.; Suryanarayanan, R.; Revcolevschi, A.; Sood, A.K. Pyrochlore “dynamic spin-ice” Pr2Sn2O7 and monoclinic Pr2Ti2O7: A comparative temperature-dependent Raman study. J. Solid State Chem. 2011, 184, 2204–2208. [Google Scholar] [CrossRef]
- Li, Y.; Kowalski, P.M.; Beridze, G.; Birnie, A.R.; Finkeldei, S.; Bosbach, D. Defect formation energies in A2B2O7 pyrochlores. Scr. Mater. 2015, 107, 18–21. [Google Scholar] [CrossRef]
- Lian, J.; Helean, K.; Kennedy, B.; Wang, L.; Navrotsky, A.; Ewing, R. Effect of structure and thermodynamic stability on the response of lanthanide stannate pyrochlores to ion beam irradiation. J. Phys. Chem. B 2006, 110, 2343–2350. [Google Scholar] [CrossRef]
- Norberg, S.T.; Hull, S.; Eriksson, S.G.; Ahmed, I.; Kinyanjui, F.; Biendicho, J.J. Pyrochlore to Fluorite Transition: The Y2(Ti1–x Zrx)2O7 (0.0≤ x≤ 1.0) System. Chem. Mater. 2012, 24, 4294–4300. [Google Scholar] [CrossRef]
- Zhang, Z.; Middleburgh, S.C.; de los Reyes, M.; Lumpkin, G.R.; Kennedy, B.J.; Blanchard, P.E.; Reynolds, E.; Jang, L.-Y. Gradual Structural Evolution from Pyrochlore to Defect-Fluorite in Y2Sn2–xZrxO7: Average vs. Local Structure. J. Phys. Chem. C 2013, 117, 26740–26749. [Google Scholar] [CrossRef]
- Ali Biswas, A.; Jana, Y. Study on the Low-Temperature Properties of Pyrochlores Gd2Hf2O7 and Gd2Zr2O7, Using Crystal-field Theory. AIP Conf. Proc. 2011, 1349, 112–1122. [Google Scholar]
- Cepeda-Sánchez, N.; Fuentes, A.; López-Cota, F.; Rodríguez-Reyes, M.; Díaz-Guillén, J. Mechanochemical synthesis and electrical properties of Gd2Hf2−xZrxO7 solid electrolytes for their use in SOFC’s. J. Appl. Electrochem. 2015, 45, 1231–1237. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, H. First principles study of dielectric and vibrational properties of pyrochlore hafnates. Solid State Sci. 2012, 14, 1405–1411. [Google Scholar] [CrossRef]
- Sevastyanov, V.G.; Simonenko, E.P.; Simonenko, N.P.; Stolyarova, V.L.; Lopatin, S.I.; Kuznetsov, N.T. Synthesis, vaporization and thermodynamic properties of superfine Nd2Hf2O7 and Gd2Hf2O7. Eur. J. Inorg. Chem. 2013, 2013, 4636–4644. [Google Scholar] [CrossRef]
- Gupta, S.K.; Abdou, M.; Ghosh, P.S.; Zuniga, J.P.; Mao, Y. Thermally Induced Disorder–Order Phase Transition of Gd2Hf2O7: Eu3+ Nanoparticles and Its Implication on Photo-and Radioluminescence. ACS Omega 2019, 4, 2779–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchard, P.E.; Clements, R.; Kennedy, B.J.; Ling, C.D.; Reynolds, E.; Avdeev, M.; Stampfl, A.P.; Zhang, Z.; Jang, L.-Y. Does local disorder occur in the pyrochlore zirconates? Inorg. Chem. 2012, 51, 13237–13244. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sheu, H.; Deng, J.; Kao, H.-C. Preparation and fluorite–pyrochlore phase transformation in Gd2Zr2O7. J. Alloy. Compd. 2009, 487, 595–598. [Google Scholar] [CrossRef]
- Popov, V.; Menushenkov, A.; Zubavichus, Y.V.; Yaroslavtsev, A.; Leshchev, D.; Kulik, E.; Bednarcik, J.; Petrunin, V.; Korovin, S.; Chernikov, R. Characteristic features of the nanocrystalline structure formation in Ln2Hf2O7(Ln= Gd, Dy) compounds. Russ. J. Inorg. Chem. 2013, 58, 1400–1407. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Navrotsky, A.; Tangeman, J.A.; Helean, K.B. Energetics of defect fluorite and pyrochlore phases in lanthanum and gadolinium hafnates. J. Am. Ceram. Soc. 2007, 90, 1171–1176. [Google Scholar] [CrossRef]
- Babu, R.; Nagarajan, K. Calorimetric measurements on rare earth pyrohafnates RE2Hf2O7 (RE= La, Eu, Gd). J. Alloy. Compd. 1998, 265, 137–139. [Google Scholar] [CrossRef]
- Pokhrel, M.; Gupta, S.K.; Wahid, K.; Mao, Y. Pyrochlore Rare-Earth Hafnate RE2Hf2O7 (RE= La and Pr) Nanoparticles Stabilized by Molten-Salt Synthesis at Low Temperature. Inorg. Chem. 2019, 58, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Simeone, D.; Thorogood, G.J.; Huo, D.; Luneville, L.; Baldinozzi, G.; Petricek, V.; Porcher, F.; Ribis, J.; Mazerolles, L.; Largeau, L.; et al. Intricate disorder in defect fluorite/pyrochlore: A concord of chemistry and crystallography. Sci. Rep. 2017, 7, 3727. [Google Scholar] [CrossRef] [PubMed]
- Martel, L.; Naji, M.; Popa, K.; Vigier, J.-F.; Somers, J. Fingerprint of local disorder in long range ordered isometric pyrochlores. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pilania, G.; Puchala, B.; Uberuaga, B.P. Distortion-stabilized ordered structures in A2BB’O7 mixed pyrochlores. NPJ Comput. Mater. 2019, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Pokhrel, M.; Brik, M.G.; Mao, Y. Particle size and crystal phase dependent photoluminescence of La2Zr2O7: Eu3+ nanoparticles. J. Am. Ceram. Soc. 2015, 98, 3192–3201. [Google Scholar] [CrossRef]
- Pokhrel, M.; Burger, A.; Groza, M.; Mao, Y. Enhance the photoluminescence and radioluminescence of La2Zr2O7: Eu3+ core nanoparticles by coating with a thin Y2O3 shell. Opt. Mater. 2017, 68, 35–41. [Google Scholar] [CrossRef]
- Wahid, K.; Pokhrel, M.; Mao, Y. Structural, photoluminescence and radioluminescence properties of Eu3+ doped La2Hf2O7 nanoparticles. J. Solid State Chem. 2017, 245, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Dasbiswas, K.; Ludwig, N.B.; Han, G.; Lee, B.; Vaikuntanathan, S.; Talapin, D.V. Stable colloids in molten inorganic salts. Nature 2017, 542, 328. [Google Scholar] [CrossRef]
- Dash, A.; Vaßen, R.; Guillon, O.; Gonzalez-Julian, J. Molten salt shielded synthesis of oxidation prone materials in air. Nat. Mater. 2019, 18, 465. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xie, H.; Wang, L.; Deng, S.; Li, J. Glass-ceramics with internally crystallized pyrochlore for the immobilization of uranium wastes. Ceram. Int. 2019, 45, 16999–17005. [Google Scholar] [CrossRef]
- Lu, X.; Shu, X.; Wang, L.; Shao, D.; Zhang, H.; Zhang, K.; Xie, Y. Heavy-ion irradiation effects on Gd2Zr2O7 ceramics bearing complex nuclear waste. J. Alloy. Compd. 2019, 771, 973–979. [Google Scholar] [CrossRef]
- Lee, W.E.; Gilbert, M.; Murphy, S.T.; Grimes, R.W. Opportunities for advanced ceramics and composites in the nuclear sector. J. Am. Ceram. Soc. 2013, 96, 2005–2030. [Google Scholar] [CrossRef]
- Petersen, M.; Hafner, J.; Marsman, M. Structural, electronic and magnetic properties of Gd investigated by DFT + U methods: Bulk, clean and H-covered (0001) surfaces. J. Phys. Condens. Matter 2006, 18, 7021–7043. [Google Scholar] [CrossRef]
- Ertas, Y.N.; Jarenwattananon, N.N.; Bouchard, L.-S. Oxide-free gadolinium nanocrystals with large magnetic moments. Chem. Mater. 2015, 27, 5371–5376. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, R. A chemical and theoretical way to look at bonding on surfaces. Rev. Mod. Phys. 1988, 60, 601. [Google Scholar] [CrossRef]
- Hughbanks, T.; Hoffmann, R. Chains of trans-edge-sharing molybdenum octahedra: Metal-metal bonding in extended systems. J. Am. Chem. Soc. 1983, 105, 3528–3537. [Google Scholar] [CrossRef]
- Ruggiero, M.T.; Erba, A.; Orlando, R.; Korter, T.M. Origins of contrasting copper coordination geometries in crystalline copper sulfate pentahydrate. Phys. Chem. Chem. Phys. 2015, 17, 31023–31029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dovesi, R.; Saunders, V.; Roetti, C.; Orlando, R.; Zicovich-Wilson, C.; Pascale, F.; Civalleri, B.; Doll, K.; Harrison, N.; Bush, I.J.U. Torino, Italy, CRYSTAL17,(2017) CRYSTAL17 User’s Manual. Available online: https://www.crystal.unito.it/Manuals/crystal17.pdf (accessed on 10 September 2019).
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange, screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.J.; Scuseria, G.E. Predicting band gaps with hybrid density functionals. J. Phys. Chem. Lett. 2016, 7, 4165–4170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Mavrin, B.N. Band and Impurity States in Dimond with the (MV)– (M = Si, Ge, Sn) Centers Based on ab Initio Calculations. J. Exp. Theor. Phys. 2018, 127, 1016–1021. [Google Scholar] [CrossRef]
- Melissen, S.T.A.G.; Labat, F.; Sautet, P.; Bahers, T.L. Electronic properties of PbX3CH3NH3 (X=Cl, Br, I) compounds for photovoltaic and photocatalytic applications. Phys. Chem. Chem. Phys. 2015, 17, 2199–2209. [Google Scholar] [CrossRef]
- BaQais, A.; Curutchet, A.; Ziani, A.; Ahsaine, H.A.; Sautet, P.; Takanabe, K.; Bahers, T.L. Bismuth Silver Oxysulfide for Photoconversion Applications: Structural and Optoelectronic Properties. Chem. Mat. 2017, 29, 8679–8689. [Google Scholar] [CrossRef] [Green Version]
- Andrae, D.; Haeussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Clickable Map of Pseudopotentials. Available online: http://www.uni-stuttgart.de/theochem/ (accessed on 10 September 2019).
- El-Kelany, K.E.; Ravoux, C.; Desmarais, J.K.; Cortona, P.; Pan, Y.; Tse, J.S.; Erba, A. Spin localization, magnetic ordering, and electronic properties of strongly correlated Ln2O3 sesquioxides(Ln=La, Ce, Pr, Nd). Phy. Rev. B 2018, 97, 245118. [Google Scholar] [CrossRef]
- Desmarais, J.K.; Erba, A.; Dovesi, R. Generalization of the periodic LCAO approach in the CRYSTAL code to g-type orbitals. Theor. Chem. Acc. 2018, 137, 28. [Google Scholar] [CrossRef]
- Munoz-Ramo, D.; Gavartin, J.L.; Shluger, A.L. Spectroscopic properties of oxygen vacancies in monoclinic HfO2 calculated with periodic and embedded cluster density functional theory. Phys. Rev. B 2007, 75, 205336. [Google Scholar] [CrossRef]
- Mahmoud, A.; Erba, A.; El-Kelany, K.E.; Rérat, M.; Orlando, R. Low-temperature phase of BaTiO3: Piezoelectric, dielectric, elastic, and photoelastic properties from ab initio simulations. Phys. Rev. B 2014, 89, 045103. [Google Scholar] [CrossRef]
- Dimakis, N.; Flor, F.A.; Navarro, N.E.; Salgado, A.; Smotkin, E.S. Adsorption of Carbon Monoxide on Platinum–Ruthenium, Platinum–Osmium, Platinum–Ruthenium–Osmium, and Platinum–Ruthenium–Osmium–Iridium Alloys. J. Phys. Chem. C 2016, 120, 10427–10441. [Google Scholar] [CrossRef]
- Dimakis, N.; Salas, I.; Gonzalez, L.; Loupe, N.; Smotkin, E.S. Electron density topological and adsorbate orbital analyses of water and carbon monoxide co-adsorption on platinum. J. Chem. Phys. 2019, 150, 024703. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Dronskowski, R.; Blöchl, P.E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Grechnev, A.; Ahuja, R.; Eriksson, O. Balanced crystal orbital overlap population—A tool for analysing chemical bonds in solids. J. Phys. Condens. Matter 2003, 15, 7751. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic population analysis on LCAO–MO molecular wave functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef] [Green Version]
- Sevast’yanov, V.G.; Simonenko, E.P.; Simonenko, N.P.; Sakharovb, K.A.; Kuznetsov, N.T. Synthesis of finely dispersed La2Zr2O7, La2Hf2O7, Gd2Zr2O7 and Gd2Hf2O7 oxides. Mendeleev Commun. 2013, 23, 17–18. [Google Scholar] [CrossRef]
- Lian, J.; Ewing, R.C.; Wang, L.M.; Helean, K.B. Ion-beam irradiation of Gd2Sn2O7 and Gd2Hf2O7 pyrochlore: Bond-type effect. J. Mater. Res. 2004, 19, 1575–1580. [Google Scholar] [CrossRef]
- Li, N.; Xiao, H.Y.; Zu, X.T.; Wang, L.M.; Ewing, R.C.; Lian, J.; Gao, F. First-principles study of electronic properties of La2Hf2O7 and Gd2Hf2O7. J. Appl. Phys. 2007, 102, 063704. [Google Scholar] [CrossRef]
- Mallia, G.; Orlando, R.; Roetti, C.; Ugliengo, P.; Dovesi, R. F center in LiF: A quantum mechanical ab initio investigation of the hyperfine interaction between the unpaired electron at the vacancy and its first seven neighbors. Phys. Rev. B. 2001, 63, 235102. [Google Scholar] [CrossRef]
- Shi, H.; Eglitis, R.; Borstel, G. Ab initio calculations of the BaF2 bulk and surface F centres. J. Phys. Condens. Matter 2006, 18, 8367. [Google Scholar] [CrossRef]
- Lichanot, A.; Orlando, R.; Mallia, G.; Merawa, M.; Dovesi, R. VOH center in magnesium oxide: An ab initio supercell study. Chem. Phys. Lett. 2000, 318, 240–246. [Google Scholar] [CrossRef]
- Govindan, K.V.; Rajagopalan, K.S.; Mathews, C.K.; Varadaraju, U.V. Thermal expansion behaviour of some rare earth oxide pyrochlores. Mater. Res. Bull. 1994, 29, 759–766. [Google Scholar]
- Lan, G.; Ouyang, B.; Song, J.J.A.M. The role of low-lying optical phonons in lattice thermal conductance of rare-earth pyrochlores: A first-principle study. Acta Mater. 2015, 91, 304–317. [Google Scholar] [CrossRef] [Green Version]
- Arai, S.; Muto, S.; Murai, J.; Sasaki, T.; Ukyo, Y.; Kuroda, K.; Saka, H. Valence change of cations in ceria-zirconia solid solution associated with redox reactions studied with electron energy-loss spectroscopy. Mater. Trans. 2004, 45, 2951–2955. [Google Scholar] [CrossRef]
- Mandal, B.P.; Garg, N.; Sharma, S.M.; Tyagi, A.K. Preparation, XRD and Raman spectroscopic studies on new compounds RE2Hf2O7 (RE=Dy, Ho, Er, Tm, Lu, Y): Pyrochlores or defect-fluorite? J. Solid State Chem. 2006, 179, 1990–1994. [Google Scholar] [CrossRef]
- Brown, S.; Gupta, H.C.; Alonso, J.A.; Martinez-Lope, M.J. Vibrational spectra and force field calculation of A2Mn2O7 (A = Y, Dy, Er, Yb) pyrochlores. J. Raman Spectrosc. 2003, 34, 240–243. [Google Scholar] [CrossRef]
- Gupta, H.C.; Brown, S.; Rani, N.; Gohel, V.B. A lattice dynamical investigation of the Raman and the infrared frequencies of the cubic A2Hf2O7 pyrochlores. J. Phys. Chem. Solids 2002, 63, 535–538. [Google Scholar] [CrossRef]
- Paul, B.; Singh, K.; Jaroń, T.; Roy, A.; Chowdhury, A. Structural properties and the fluorite–pyrochlore phase transition in La2Zr2O7: The role of oxygen to induce local disordered states. J. Alloy. Compd. 2016, 686, 130–136. [Google Scholar] [CrossRef]
- Garbout, A.; Kallel- Kchaou, N.; Férid, M. Relationship between the structural characteristics and photoluminescent properties of LnEuTi2O7 (Ln=Gd and Y) pyrochlores. J. Lumin. 2016, 169, 359–366. [Google Scholar] [CrossRef]
- Vandenborre, M.T.; Husson, E.; Chatry, J.P.; Michel, D. Rare-earth titanates and stannates of pyrochlore structure; vibrational spectra and force fields. J. Raman Spectrosc. 1983, 14, 63–71. [Google Scholar] [CrossRef]
- Gardner, J.S.; Gingras, M.J.; Greedan, J.E. Magnetic pyrochlore oxides. Rev. Mod. Phys. 2010, 82, 53. [Google Scholar] [CrossRef]
- Williams, A.R.; Zeller, R.; Moruzzi, V.L.; Gelatt, C.D.; Kubler, J. Covalent magnetism: An alternative to the Stoner model. J. Appl. Phys. 1981, 52, 2067–2069. [Google Scholar] [CrossRef]
- Stoner, E.C. Collective electron ferromagnetism in metals and alloys. J. Phys. Radium 1951, 12, 372–388. [Google Scholar] [CrossRef] [Green Version]
- Zeller, R. Spin-polarized dft calculations and magnetism. Do It Yours. 2006, 31, 419–445. [Google Scholar]
- Gruber, C.; Bedolla, P.O.; Mohn, P. Covalent magnetism and magnetic impurities. J. Phys. Condens. Matter 2013, 25, 186002. [Google Scholar] [CrossRef] [PubMed]
- Ammeter, J.H.; Bürgi, H.-B.; Thibeault, J.C.; Hoffmann, R. Counterintuitive Orbital Mixing in Semiempirical and ab Initio Molecular Orbital Calculations. J. Am. Chem. Soc. 1978, 100, 3686–3692. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (GHO-650 °C, GHO-1000 °C, GHO-1250 °C and GHO-1500 °C) are available from the authors if requested. |









| OP-GHO | Lattice Parameters | Distances | |||||
|---|---|---|---|---|---|---|---|
| a | x | Hf-Gd | Gd-O8b | Gd-O48f | Hf-O8b | Hf-O48f | |
| (Å) | |||||||
| O8a ghost atoms calc. | 10.473 | 0.3368 | 3.702 | 2.267 | 2.519 | 4.342 | 2.063 |
| (10.427) | (0.3767) | (3.687) | (4.323) | (2.267) | (2.258) | (2.248) | |
| O8a vacant calc. | 10.397 | 0.3377 | 3.676 | 2.251 | 2.495 | 4.310 | 2.052 |
| (10.254) | (0.3751) | (3.625) | (4.261) | (2.227) | (2.226) | (2.224) | |
| Other calc. [79] | 10.55 | 0.337 | |||||
| [74] | 10.53 | 0.3367 | 2.2798 | 2.5339 | 2.0734 | ||
| Experimental [78] | 10.49 | ||||||
| Atom Pair | GHO | |
|---|---|---|
| O8a via Ghost Atoms | O8avacant | |
| -COHP | ||
| Hf-Gd | –0.002 (–0.016) | <0.001 (−0.014) |
| Gd-O8b | −0.086 (<0.001) | −0.088 (−0.006) |
| Gd-O48f | −0.023 (−0.062) | −0.024 (−0.170) |
| Hf-O8b | <0.001 (−0.027) | <0.001 (−0.036) |
| Hf-O48f | −0.107 (−0.031) | −0.111 (−0.068) |
| COOP | ||
| Hf-Gd | < 0.001 (<0.001) | <0.001 (−0.009) |
| Gd-O8b | −0.042 (<0.001) | −0.043 (−0.033) |
| Gd-O48f | −0.010 (−0.027) | −0.011 (−0.103) |
| Hf-O8b | <0.001 (0.020) | <0.001 (−0.048) |
| Hf-O48f | −0.041 (0.012) | −0.045 (−0.067) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokhrel, M.; Dimakis, N.; Dannangoda, C.; Gupta, S.K.; Martirosyan, K.S.; Mao, Y. Structural Evolution and Magnetic Properties of Gd2Hf2O7 Nanocrystals: Computational and Experimental Investigations. Molecules 2020, 25, 4847. https://doi.org/10.3390/molecules25204847
Pokhrel M, Dimakis N, Dannangoda C, Gupta SK, Martirosyan KS, Mao Y. Structural Evolution and Magnetic Properties of Gd2Hf2O7 Nanocrystals: Computational and Experimental Investigations. Molecules. 2020; 25(20):4847. https://doi.org/10.3390/molecules25204847
Chicago/Turabian StylePokhrel, Madhab, Nicholas Dimakis, Chamath Dannangoda, Santosh K. Gupta, Karen S. Martirosyan, and Yuanbing Mao. 2020. "Structural Evolution and Magnetic Properties of Gd2Hf2O7 Nanocrystals: Computational and Experimental Investigations" Molecules 25, no. 20: 4847. https://doi.org/10.3390/molecules25204847