A Simplified Method of Synthesis to Obtain Zwitterionic Cellulose under Mild Conditions with Active Ionic Moieties
Abstract
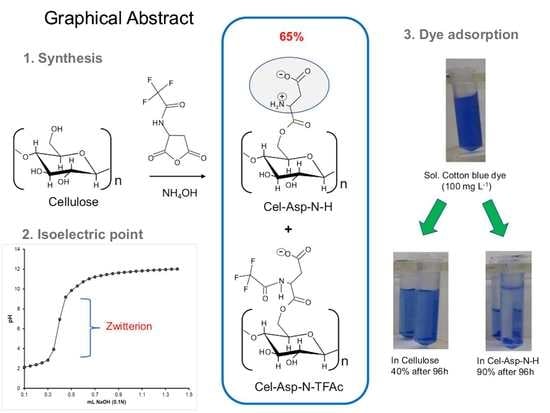
:1. Introduction
2. Results and Discussion
2.1. Spectroscopic Characterization of the Cel-Asp Samples
2.2. Evaluation of the Functionalization and Deprotection Degrees in Aspartate-Modified Celluloses
2.3. Zwitterion Character in the Cel-Asp-N-H
2.4. Availability of the Zwitterionic Moieties in Cel-Asp-N-H by a Dye Adsorption Test
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Zwitterionic Celluloses from Aspartic Anhydrides
3.3. Characterization
3.4. Determination of the Degree of Functionalization in Celluloses
3.5. Determination of the N-Deprotection Degree of the Aspartate
3.6. Zwitterion Character Evaluation
3.7. Availability of the Zwitterionic Moieties by Dye Adsorption Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clarkd, J.H.; MacLachlang, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Hon, D.N.-S. Cellulose and its Derivatives: Structures, Reactions, and Medical Uses, In Polysaccharides in Medical Applications; Dumitru, S., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 87–105. [Google Scholar]
- Lai, Y. Chemical modification of cellulose, hemicelluloses and lignins. In Chemical Modification of Lignocellulosic Materials; Hon, D.N.-S., Ed.; Marcel Dekker Inc.: New York, NY, USA; Basel, Switzerland; Hong Kong, China, 1996; pp. 97–127. [Google Scholar]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsenf, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.J.; Glasser, W.G. The role of the novel solvents and solution complexes for the preparation of highly engineering cellulose derivatives. In Cellulose Derivatives: Modification, Characterization and Nanoestructures; Heinze, T.J., Glasser, W.G., Eds.; American Chemical Society: Washington, DC, USA, 1998; pp. 2–18. [Google Scholar]
- Liebert, T. Cellulose Solvents-Remarkable History, Bright Future. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; Symposium Series; American Chemical Society: Washington, DC, USA, 2010; pp. 3–54. [Google Scholar]
- Otto, E.; Schempp, W.; Krause, T. Production of cationic cellulose of high substitution grades in the system lithium-Chloride/dimethylacetamide. Papier 1989, 43, 694–699. [Google Scholar]
- Song, Y.; Zhou, J.; Zhang, L.; Wu, X. Homogenous modification of cellulose with acrylamide in NaOH/urea aqueous solutions. Carbohydr. Polym. 2008, 73, 18–25. [Google Scholar] [CrossRef]
- Anastas, P.; Eghabali, N. Green Chemistry: Principles and Practice. R. Soc. Chem. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Sousa, K.S.; Silva Filho, E.C.; Airoldi, C. Ethylenesulfide as a useful agent for incorporation into the biopolymer chitosan in a solvent-free reaction for use in cation removal. Carbohydr. Res. 2009, 344, 1716–1723. [Google Scholar] [CrossRef]
- Knaus, S.; Bauer-Heim, B. Synthesis and properties of anionic cellulose ethers: Influence of functional groups and molecular weight on flowability of concrete. Carbohydr. Polym. 2003, 53, 383–394. [Google Scholar] [CrossRef]
- Petroudy, S.R.D.; Syveruda, K.; Chinga-Carrasco, G.; Ghasemain, A.; Resalati, H. Effects of bagasse microfibrillated cellulose and cationic polyacrylamide on key properties of bagasse paper. Carbohydr. Polym. 2014, 99, 311–318. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Zaman, M.; Mohammed, N.; Brinatti, C.; Batmaz, R.; Berry, R.; Loh, W.; Tam, K.C. Synthesis of amine functionalized cellulose nanocrystals: Optimization and characterization. Carbohydr. Res. 2015, 409, 48–55. [Google Scholar] [CrossRef]
- Salimi, H.; Aryanasab, F.; Banazadeh, A.R.; Shabanian, M.; Seidi, F. Designing syntheses of cellulose and starch derivatives with basic or cationic N-functions: Part I—cellulose derivatives. Polym. Adv. Technol. 2016, 27, 5–32. [Google Scholar] [CrossRef]
- Araújo, A.C.; Nakhai, A.; Ruda, M.; Slättegård, R.; Gatenholm, P.; Brumer, H. A general route to xyloglucan-peptide conjugates for the activation of cellulose surfaces. Carbohydr. Res. 2012, 354, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Genco, T.; Petzold-Welcke, K.; Wondraczek, H. Synthesis and characterization of aminocellulose sulfates as novel ampholytic polymers. Cellulose 2012, 19, 1305–1313. [Google Scholar] [CrossRef]
- Li, X.; Cao, Y.; Kang, G.; Yu, H.; Jie, X.; Yuan, Q. Surface Modification of Polyamide Nanofiltration Membrane by Grafting Zwitterionic Polymers to Improve the Antifouling Property. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, X.; Brault, N.; Keefe, A.; Han, X.; Deng, Y.; Xu, J.; Yu, Q.; Jiang, S. Cellulose Paper Sensors Modified with Zwitterionic Poly(carboxybetaine) for Sensing and Detection in Complex Media. Anal. Chem. 2014, 86, 2871–2875. [Google Scholar] [CrossRef]
- Delgado, E.; López-Dellamary, F.; Allan, G.; Andrade, A.; Contreras, H.; Regla, H.; Cresson, T. Zwitterion modification of fibres: Effect of fibre flexibility on wet strength of paper. J. Pulp Pap. Sci. 2004, 30, 141–144. [Google Scholar]
- Manríquez, R.; López-Dellamary, F.; Frydel, J.; Emmler, T.; Breitzke, H.; Buntkowsky, G.; Limbach, H.-H.; Shenderovich, I.G. Solid-state NMR studies of aminocarboxylic salt bridges in L-lysine modified cellulose. J. Phys. Chem. B 2009, 113, 934–940. [Google Scholar] [CrossRef]
- Allan, G.; Delgado, E.; López-Dellamary, F. A new interfibre system for paper involving zwitterions. Pulp Pap. Fund. Res. Soc. 1983, 2, 1101–1138. [Google Scholar]
- Laureano-Anzaldo, C.M.; Haro-Mares, N.B.; Meza-Contreras, J.C.; Robledo-Ortíz, J.R.; Manríquez-González, R. Chemical modification of cellulose with zwitterion moieties used in the uptake of red Congo dye from aqueous media. Cellulose 2019, 26, 9207–9227. [Google Scholar] [CrossRef]
- Liu, H.; Taylor, L.S.; Edgar, K.J. The role of polymers in oral bioavailability enhancement; a review. Polymer 2015, 77, 399–415. [Google Scholar] [CrossRef] [Green Version]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Cuculo, J.A. Modifying Cellulosic Fabric with Dicarboxylic Acids to Impart Water-Dispersibility. U.S. Patent No. 3,671,184, 20 June 1972. U.S. Patent and Trademark Office, Washington, DC, USA. [Google Scholar]
- Kretzschmar, G. Polysacharide Aspartate. European Patent No. 1 065 217 A1, 3 January 2001. European Patent Office, Frankfurt am Main DE, Germany. [Google Scholar]
- Karimi, K.; Taherzadeh, M.J. A critical review on analysis in pretreatment of lignocelluloses: Degree of polymerization, adsorption/desorption, and accessibility. Bioresour. Technol. 2016, 203, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garside, P.; Wyeth, P. Identification of cellulose fibres by FTIR spectroscopy. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Poletto, M.; Ornaghi Júnior, H.L.; Zattera, A.J. Native cellulose: Structure, characterization and thermal properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, A. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Bio. 2000, 74, 141–173. [Google Scholar] [CrossRef]
- Gonzalez-McQuire, R.; Chane-Ching, J.Y.; Vignaud, E.; Lebugle, A.; Mann, S. Synthesis and characterization of amino acid-functionalized hydroxyapatite nanorods. J. Mater. Chem. 2004, 14, 2277–2281. [Google Scholar] [CrossRef]
- Sebben, D.; Pendleton, P. Infrared spectrum analysis of the dissociated states of simple amino acids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 706–712. [Google Scholar] [CrossRef]
- Meza-Contreras, J.C.; Manríquez-González, R.; Gutiérrez-Ortega, J.A.; González-García, Y. XRD and solid state 13C-NMR evaluation of the crystallinity enhancement of 13C-labeled bacterial cellulose biosynthesized by Komagataeibacter xylinus under different stimuli: A comparative strategy of analyses. Carbohydr. Res. 2018, 461, 51–59. [Google Scholar] [CrossRef]
- Mittal, A.; Katahira, R.; Himmel, M.E.; Johnson, D.K. Effects of alkaline or liquid-ammonia treatment on crystalline cellulose: Changes in crystalline structure and effects on enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gould, J.A.; Jones, J.T.A.; Bacsa, J.; Khimyak, Y.Z.M.; Rosseinsky, J.A. A homochiral three-dimensional zinc aspartate framework that displays multiple coordination modes and geometries. RSC Chem. Comm. 2010, 46, 2793–2795. [Google Scholar] [CrossRef]
- Sahoo, L.; Singhamahapatra, A.; Ramkumar, V.; Loganathan, D. Regioselective opening of unsymmetrical cyclic anhydrides: Synthesis of N-glycosylated isoasparagine and isoglutamine conjugates. RSC Adv. 2014, 4, 22042–22047. [Google Scholar] [CrossRef]
- Hon, D.N.-S.; Yan, H. Cellulose Furoate. I. Synthesis in homogeneous and heterogeneous systems. J. Appl. Polym. Sci. 2001, 81, 2649–2655. [Google Scholar] [CrossRef]
- Yoshida, Y.; Heux, L.; Isogai, A. Heterogeneous reaction between cellulose and alkyl ketene dimer under solvent-free conditions. Cellulose 2012, 19, 1667–1676. [Google Scholar] [CrossRef]
- Junka, K.; Filpponen, I.; Johansson, L.-S.; Kontturi, E.; Rojas, O.J.; Laine, J. A method for the heterogeneous modification of nanofibrillar cellulose in aqueous media. Carbohydr. Polym. 2014, 100, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.O. Regiocontrol in cellulose chemistry: Principles and examples of etherification and esterification. In Cellulose Derivatives: Modification, Characterization and Nanoestructures; Heinze, T.J., Glasser, W.G., Eds.; American Chemical Society: Washington, DC, USA, 1998; pp. 19–37. [Google Scholar]
- Edwards, J.V.; Provost, N.T.; Condon, B.; French, A.; Wu, Q. Inmobilization of lysozyme-cellulose amide-linked conjugates in cellulose I and II cotton nanocrystalline preparations. Cellulose 2012, 19, 495–506. [Google Scholar] [CrossRef]
- Isidro-Llobet, A.; Álvarez, M.; Albericio, F. Amino acid-protectig groups. Chem. Rev. 2009, 109, 2455–2504. [Google Scholar] [CrossRef] [Green Version]
- Drnovšek, T.; Perdih, A. Selective staining as a tool for wood fibre characterization. Dyes Pigments 2005, 67, 197–206. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Chandra, R.P.; Dogu, D.; van Velzen, S.T.J. Analytical staining of cellulosic materials: A review. BioResources 2019, 14, 7387–7464. [Google Scholar]
- Ho, F.F.-L.; Kohler, R.R.; Ward, G.A. Determination of molar substitution and degree of substitution of hydroxypropyl cellulose by nuclear magnetic resonance spectrometry. Anal. Chem. 1972, 44, 178–181. [Google Scholar] [CrossRef]
- Metz, G.; Ziliox, M.S.; Smith, O. Towards quantitative CP-MAS NMR. Solid State Nucl. Magn. Reson. 1996, 7, 155–160. [Google Scholar] [CrossRef]
- Dominiak, K.; Ebeling, H.; Kunze, J.; Fink, H.-P. 13C-NMR spectroscopical investigations of the substituent distribution in cellulose xanthates. Lenzinger Ber. 2011, 89, 132–141. [Google Scholar]
- Gaborieau, M.; Nebhani, L.; Graf, R.; Barner, L.; Barner-Kowollik, C. Accessing Quantitative Degrees of Functionalization on Solid Substrates via Solid-State NMR Spectroscopy. Macromolecules 2010, 43, 3868–3875. [Google Scholar] [CrossRef]
- Volkert, B.; Lehmann, A.; Hettrich, K. Novel cellulose and starch-based materials. Cellul. Chem. Technol. 2014, 48, 425–444. [Google Scholar]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to Agricultural and Biomedical Sciences. J. Agric. Food Chem. 2004, 52, 385–406. [Google Scholar] [CrossRef]
- Sneddon, H.F. Safety first. Green Chem. 2016, 18, 5082–5085. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |








| Modified Celluloses | 13C NMR Areas | % of mod. by NMRa | Elemental Analysis | % of mod. by AE | % of Deprotection in Asp. | ||
|---|---|---|---|---|---|---|---|
| C1 | Cα | %C | %N | ||||
| Cel-Asp-N-TFAc | 58.8 | 10.4 | 18% | 39.756 | 1.402 | 21% | 13% |
| Cel-Asp-N-Cbz | 52.0 | 7.7 | 15% | 44.752 | 1.398 | 17% | 0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haro-Mares, N.B.; Meza-Contreras, J.C.; López-Dellamary Toral, F.A.; González-Cruz, R.; Silva-Guzmán, J.A.; Manríquez-González, R. A Simplified Method of Synthesis to Obtain Zwitterionic Cellulose under Mild Conditions with Active Ionic Moieties. Molecules 2020, 25, 3065. https://doi.org/10.3390/molecules25133065
Haro-Mares NB, Meza-Contreras JC, López-Dellamary Toral FA, González-Cruz R, Silva-Guzmán JA, Manríquez-González R. A Simplified Method of Synthesis to Obtain Zwitterionic Cellulose under Mild Conditions with Active Ionic Moieties. Molecules. 2020; 25(13):3065. https://doi.org/10.3390/molecules25133065
Chicago/Turabian StyleHaro-Mares, Nadia B., Juan C. Meza-Contreras, Fernando A. López-Dellamary Toral, Ricardo González-Cruz, José A. Silva-Guzmán, and Ricardo Manríquez-González. 2020. "A Simplified Method of Synthesis to Obtain Zwitterionic Cellulose under Mild Conditions with Active Ionic Moieties" Molecules 25, no. 13: 3065. https://doi.org/10.3390/molecules25133065