The Study on the hERG Blocker Prediction Using Chemical Fingerprint Analysis
Abstract
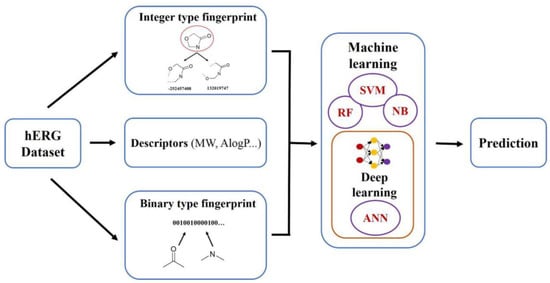
:1. Introduction
2. Results
2.1. Dataset Splitting
2.2. Model Building and Evaluation
2.3. External Validation
3. Discussion
4. Materials and Methods
4.1. Dataset
4.2. Fingerprint Calculation
4.3. Model Building
4.4. Model Evaluation
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Priest, B.; Bell, I.M.; Garcia, M. Role of hERG potassium channel assays in drug development. Channels 2008, 2, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-M.; Yu, M.-S.; Kazmi, S.R.; Oh, S.Y.; Rhee, K.-H.; Bae, M.-A.; Lee, B.H.; Shin, D.-S.; Oh, K.-S.; Ceong, H. Computational determination of hERG-related cardiotoxicity of drug candidates. BMC Bioinform. 2019, 20, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laverty, H.; Benson, C.; Cartwright, E.; Cross, M.; Garland, C.; Hammond, T.; Holloway, C.; McMahon, N.; Milligan, J.; Park, B. How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines? Br. J. Pharmacol. 2011, 163, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Cantilena, L., Jr.; Koerner, J.; Temple, R.; Throckmorton, D. OIII-A-1: FDA evaluation of cardiac repolarization data for 19 drugs and drug candidates. Clin. Pharmacol. Ther. 2006, 79, P29. [Google Scholar] [CrossRef]
- Fernandez, D.; Ghanta, A.; Kauffman, G.W.; Sanguinetti, M.C. Physicochemical features of the HERG channel drug binding site. J. Biol. Chem. 2004, 279, 10120–10127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; MacKinnon, R. Cryo-EM structure of the open human ether-à-go-go-related K+ channel hERG. Cell 2017, 169, 422–430.e10. [Google Scholar] [CrossRef] [Green Version]
- Saxena, P.; Zangerl-Plessl, E.-M.; Linder, T.; Windisch, A.; Hohaus, A.; Timin, E.; Hering, S.; Stary-Weinzinger, A. New potential binding determinant for hERG channel inhibitors. Sci. Rep. 2016, 6, 24182. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.-l.; Lu, J.; Lu, Y.; Zheng, M.-y.; Luo, X.-m.; Zhu, W.-l.; Jiang, H.-l.; Chen, K.-x. Novel Bayesian classification models for predicting compounds blocking hERG potassium channels. Acta Pharmacol. Sin. 2014, 35, 1093. [Google Scholar] [CrossRef]
- Jia, L.; Sun, H. Support vector machines classification of hERG liabilities based on atom types. Bioorg. Med. Chem 2008, 16, 6252–6260. [Google Scholar] [CrossRef]
- Song, M.; Clark, M. Development and evaluation of an in silico model for hERG binding. J. Chem. Inf. Model. 2006, 46, 392–400. [Google Scholar] [CrossRef]
- Chavan, S.; Abdelaziz, A.; Wiklander, J.G.; Nicholls, I.A. A k-nearest neighbor classification of hERG K+ channel blockers. J. Comput. Aided Mol. Des. 2016, 30, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H. An accurate and interpretable Bayesian classification model for prediction of hERG liability. Chem. Med. Chem. 2006, 1, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.; Cai, C.; Xue, Y.; Chen, Y. Prediction of torsade-causing potential of drugs by support vector machine approach. Toxicol. Sci. 2004, 79, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Marchese Robinson, R.L.; Glen, R.C.; Mitchell, J.B. Development and comparison of hERG blocker classifiers: Assessment on different datasets yields markedly different results. Mol. Inform. 2011, 30, 443–458. [Google Scholar] [CrossRef]
- Kim, J.H.; Chae, C.H.; Kang, S.M.; Lee, J.Y.; Lee, G.N.; Hwang, S.H.; Kang, N.S. The predictive QSAR model for hERG inhibitors using Bayesian and random forest classification method. Bull. Korean Chem. Soc. 2011, 32, 1237. [Google Scholar] [CrossRef] [Green Version]
- Polak, S.; Wiśniowska, B.; Ahamadi, M.; Mendyk, A. Prediction of the hERG potassium channel inhibition potential with use of artificial neural networks. Appl. Soft Comput. 2011, 11, 2611–2617. [Google Scholar] [CrossRef]
- Cai, C.; Guo, P.; Zhou, Y.; Zhou, J.; Wang, Q.; Zhang, F.; Fang, J.; Cheng, F. Deep Learning-Based Prediction of Drug-Induced Cardiotoxicity. J. Chem. Inf. Model. 2019, 59, 1073–1084. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Wang, Y.; Fan, Y.; Zhu, L.; Yang, Y.; Chen, X.; Lu, T.; Chen, Y.; Liu, H. Prediction of hERG K+ channel blockage using deep neural networks. Chem. Biol. Drug Des. 2019, 94, 1973–1985. [Google Scholar] [CrossRef]
- Drwal, M.N.; Siramshetty, V.B.; Banerjee, P.; Goede, A.; Preissner, R.; Dunkel, M. Molecular similarity-based predictions of the Tox21 screening outcome. Front. Environ. Sci. 2015, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- Myint, K.-Z.; Wang, L.; Tong, Q.; Xie, X.-Q. Molecular fingerprint-based artificial neural networks QSAR for ligand biological activity predictions. Mol. Pharm. 2012, 9, 2912–2923. [Google Scholar] [CrossRef]
- Myint, K.Z.; Xie, X.-Q. Artificial Neural Networks; Springer: New York, NY, USA, 2015; pp. 149–164. [Google Scholar]
- Fan, T.; Sun, G.; Zhao, L.; Cui, X.; Zhong, R. QSAR and classification study on prediction of acute oral toxicity of N-nitroso compounds. Int. J. Mol. Sci. 2018, 19, 3015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-de Gortari, E.; García-Jacas, C.R.; Martinez-Mayorga, K.; Medina-Franco, J.L. Database fingerprint (DFP): An approach to represent molecular databases. J. Cheminformat. 2017, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Rogers, D.; Hahn, M. Extended-connectivity fingerprints. J. Chem. Inf. Model. 2010, 50, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, T.; Wang, B.; Gao, Y.; Liu, P.; Li, M.; Du, L. Astemizole-based turn-on fluorescent probes for imaging hERG potassium channel. Med. Chem. Comm. 2019, 10, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, Z.; Shi, Z.-C.; He, S.; Lai, Z.; Cernak, T.A.; Vachal, P.; Liu, M.; Liu, J.; Hong, Q. Accelerating the discovery of DGAT1 inhibitors through the application of parallel medicinal chemistry (PMC). Bioorgan. Med. Chem. Lett. 2019, 29, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Okumu, A.; Dellos-Nolan, S.; Li, Z.; Karmahapatra, S.; English, A.; Yalowich, J.C.; Wozniak, D.J.; Mitton-Fry, M.J. Synthesis and anti-staphylococcal activity of novel bacterial topoisomerase inhibitors with a 5-amino-1, 3-dioxane linker moiety. Bioorgan. Med. Chem. Lett. 2018, 28, 2477–2480. [Google Scholar] [CrossRef]
- Bagdanoff, J.T.; Chen, Z.; Acker, M.; Chen, Y.-N.; Chan, H.; Dore, M.; Firestone, B.; Fodor, M.; Fortanet, J.; Hentemann, M. Optimization of Fused Bicyclic Allosteric SHP2 Inhibitors. J. Med. Chem. 2019, 62, 1781–1792. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, T.; Li, Q.; Li, M.; Du, L. Aggregation-Induced Emission: Lighting Up hERG Potassium Channel. Front. Chem. 2019, 7, 54. [Google Scholar] [CrossRef]
- Xuan, S.; Liang, H.; Wang, Z.; Yan, A. Classification of blocker and non-blocker of hERG potassium ion channel using a support vector machine. Sci. China Chem. 2013, 56, 1413–1423. [Google Scholar] [CrossRef]
- Du, F.; Babcock, J.J.; Yu, H.; Zou, B.; Li, M. Global analysis reveals families of chemical motifs enriched for hERG inhibitors. PLoS ONE 2015, 10, e0118324. [Google Scholar] [CrossRef] [Green Version]
- Mendez, D.; Gaulton, A.; Bento, A.P.; Chambers, J.; De Veij, M.; Félix, E.; Magariños, M.P.; Mosquera, J.F.; Mutowo, P.; Nowotka, M. ChEMBL: Towards direct deposition of bioassay data. Nucleic Acid Res. 2019, 47, D930–D940. [Google Scholar] [CrossRef] [PubMed]
- Guha, R. Chemical informatics functionality in R. J. Stat. Softw 2007, 18, 1–16. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/.
- David, M.; Evgenia, D.; Kurt, H.; Andreas, W.; Friedrich, L. e1071: Misc Functions of the Department of Statistics, Probability Theory Group (Formerly: E1071), TU Wien. R Package Version 1.7-3. 2019. Available online: https://CRAN.R-project.org/package=e1071 (accessed on 31 May 2020).
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Erin, L.; Navdeep, G.; Spencer, A.; Anqi, F.; Arno, C.; Cliff, C.; Tom, K.; Tomas, N.; Patrick, A.; Michal, K.; et al. h2o: R Interface for the ‘H2O’ Scalable Machine Learning Platform. R Package Version 3.28.1.2. 2020. Available online: https://github.com/h2oai/h2o-3 (accessed on 31 May 2020).
Sample Availability: Samples of the compounds are not available from the authors. |




| Source | Set | Compounds | Active Compounds | Inactive Compounds |
|---|---|---|---|---|
| ChEMBL database (Total compounds: 5252) | Training set | 3991 | 1201 | 2790 |
| Test set | 998 | 278 | 720 | |
| Ext-1 | 263 | 67 | 196 | |
| Recent research papers (Total compounds: 47) | Ext-2 | 47 | 18 | 29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.-E.; Balupuri, A.; Kang, N.S. The Study on the hERG Blocker Prediction Using Chemical Fingerprint Analysis. Molecules 2020, 25, 2615. https://doi.org/10.3390/molecules25112615
Choi K-E, Balupuri A, Kang NS. The Study on the hERG Blocker Prediction Using Chemical Fingerprint Analysis. Molecules. 2020; 25(11):2615. https://doi.org/10.3390/molecules25112615
Chicago/Turabian StyleChoi, Kwang-Eun, Anand Balupuri, and Nam Sook Kang. 2020. "The Study on the hERG Blocker Prediction Using Chemical Fingerprint Analysis" Molecules 25, no. 11: 2615. https://doi.org/10.3390/molecules25112615