The Effects of 5,6,7,8,3′,4′-Hexamethoxyflavone on Apoptosis of Cultured Human Choriocarcinoma Trophoblast Cells
Abstract
:1. Introduction
2. Results
2.1. Cellular Uptake of NOB
2.2. The Effect of NOB on the Cell Morphology of BeWo Cells
2.3. The Effect of NOB on the Viability of BeWo Cells
2.4. The Effect of NOB on Cell Cycle Distribution of BeWo Cells
2.5. The Effect of NOB on the Apoptosis of BeWo Cells
2.6. The Effect of NOB on the Expression of BCL2 Family Proteins in BeWo Cells
2.7. The Effect of NOB on Expression of p53-Related Proteins in BeWo Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
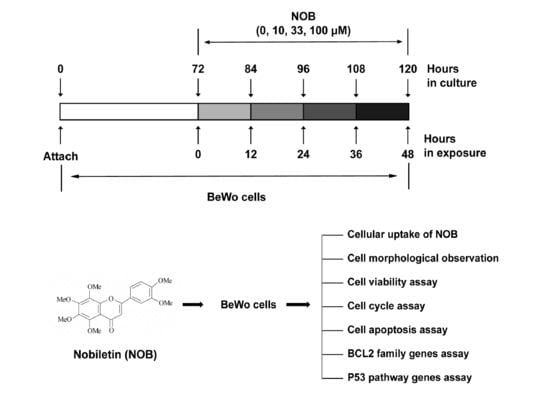
4.3. Experiment Protocol
4.4. Cellular Uptake of NOB
4.5. Cell Morphological Observation
4.6. Cell Viability Assay
4.7. Cell Cycle Assay
4.8. Cell Apoptosis Assay
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BeWo cells | Human choriocarcinoma trophoblast cells |
| Cl-PARP | Cleaved PARP |
| CU | Cellular uptake |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal bovine serum |
| NOB | Nobiletin |
| PI | Propidium iodide |
| PMSF | Phenylmethylsulfonyl fluoride |
References
- Shan, Y. Comprehensive Utilization of Citrus by-Products; Chemical Industry Press: Beijing, China, 2016; pp. 6–9, 54–56. [Google Scholar]
- He, Z.; Li, X.; Chen, H.; He, K.; Liu, Y.; Gong, J.; Gong, J. Nobiletin attenuates lipopolysaccharide/Dgalactosamine induced liver injury in mice by activating the Nrf2 antioxidant pathway and subsequently inhibiting NF-κB mediated cytokine production. Mol. Med. Rep. 2016, 14, 5595–5600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Wei, W.Y.; Yang, Z.; Che, Y.; Jin, Y.G.; Liao, H.H.; Wang, S.S.; Deng, W.; Tang, Q.Z. Nobiletin, a polymethoxy flavonoid, protects against cardiac hypertrophy induced by pressure-overload via inhibition of NAPDH oxidases and endoplasmic reticulum stress. Cell. Physiol. Biochem. 2017, 42, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, R.; Wang, X.; Liu, F.; Zhao, J.; Yao, Q.; Zhi, W.; He, Z.; Niu, X. Nobiletin-ameliorated lipopolysaccharide-induced inflammation in acute lung injury by suppression of NF-κB pathway in vivo and vitro. Inflammation 2018, 41, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Lee, D.R.; Choi, B.K.; Kim, H.S.; Yang, S.H.; Suh, J.W.; Kim, K.S. Protective effects of a polymethoxy flavonoids-rich citrus aurantium peel extract on liver fibrosis induced by bile duct ligation in mice. Asian Pac. J. Trop. Med. 2016, 9, 1158–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Chen, A.Y.; Huang, H.; Ye, X.; Rollyson, W.D.; Perry, H.E.; Brown, K.C.; Rojanasakul, Y.; Rankin, G.O.; Dasgupta, P.; et al. The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the Akt pathway. Int. J. Oncol. 2015, 46, 2629–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, S.Y.; Hsieh, M.J.; Chen, C.J.; Yang, S.F.; Chen, M.K. Nobiletin inhibits invasion and migration of human nasopharyngeal carcinoma cell lines by involving ERK1/2 and transcriptional inhibition of MMP-2. Expert Opin. Ther. Targets 2015, 19, 307–320. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Roweth, H.; Ali, M.S.; Unsworth, A.J.; Stainer, A.R.; Flora, G.D.; Crescente, M.; Jones, C.I.; Moraes, L.A.; Gibbins, J.M. Pharmacological actions of nobiletin in the modulation of platelet function. Br. J. Pharmacol. 2015, 172, 413–4145. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.R.; Yang, L.; Zhen, J.; Zhao, Y.; Lu, Z.N. Nobiletin protects PC12 cells from ERS-induced apoptosis in OGD/R injury via activation of the PI3K/AKT pathway. Exp. Ther. Med. 2018, 16, 1470–1476. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.P.; Guo, H.; Wang, X.B. Nobiletin (NOB) suppresses autophagic degradation via over-expressing AKT pathway and enhances apoptosis in multidrug-resistant SKOV3/TAX ovarian cancer cells. Biomed. Pharmacother. 2018, 103, 29–37. [Google Scholar] [CrossRef]
- Moon, J.Y.; Cho, S.K. Nobiletin induces protective autophagy accompanied by ER-stress mediated apoptosis in human gastric cancer SNU-16 cells. Molecules 2016, 21, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goan, Y.G.; Wu, W.T.; Liu, C.I.; Neoh, C.A.; Wu, Y.J. Involvement of mitochondrial dysfunction, endoplasmic reticulum stress, and the PI3K/AKT/mTOR pathway in nobiletin-induced apoptosis of human bladder cancer cells. Molecules 2019, 24, 2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Zhang, Z.; Jiang, G.; Sun, H.; Yu, D. Nobiletin sensitizes colorectal cancer cells to oxaliplatin by PI3K/Akt/MTOR pathway. Front. Biosci. 2019, 24, 303–312. [Google Scholar]
- Takii, M.; Kaneko, Y.K.; Akiyama, K.; Aoyagi, Y.; Tara, Y.; Asakawa, T.; Inai, M.; Kan, T.; Nemoto, K.; Ishikawa, T. Insulinotropic and anti-apoptotic effects of nobiletin in INS-1D beta-cells. J. Funct. Foods 2017, 30, 8–15. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, H.; Li, Y.; Lu, X. Nobiletin suppresses oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Eur. J. Pharmacol. 2019, 854, 48–53. [Google Scholar] [CrossRef]
- Keshtkar, S.; Kaviani, M.; Jabbarpour, Z.; Geramizadeh, B.; Motevaseli, E.; Nikeghbalian, S.; Shamsaeefar, A.; Motazedian, N.; Al-Abdullah, I.H.; Ghahremani, M.H.; et al. Protective effect of nobiletin on isolated human islets survival and function against hypoxia and oxidative stress-induced apoptosis. Sci. Rep. 2019, 9, 11701. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Lim, W.; Bazer, F.W.; Song, G. Avobenzone suppresses proliferative activity of human trophoblast cells and induces apoptosis mediated by mitochondrial disruption. Reprod. Toxicol. 2018, 81, 50–57. [Google Scholar] [CrossRef]
- Lanoix, D.; Lacasse, A.A.; Reiter, R.J.; Vaillancourt, C. Melatonin: The smart killer: The human trophoblast as a model. Mol. Cell. Endocrinol. 2012, 348, 1–11. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Salustiano, E.M.A.; Ruano, R.; Markus, R.P.; Vaillancourt, C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J. Pineal Res. 2018, 65, e12520. [Google Scholar] [CrossRef]
- Kohan-Ghadr, H.R.; Kilburn, B.A.; Kadam, L.; Johnson, E.; Kolb, B.L.; Rodriguez-Kovacs, J.; Hertz, M.; Armant, D.R.; Drewlo, S. Rosiglitazone augments antioxidant response in the human trophoblast and prevents apoptosisdagger. Biol. Reprod. 2019, 100, 479–494. [Google Scholar] [CrossRef]
- Yang, A.; Xiao, X.H.; Wang, Z.L.; Wang, Z.Y.; Wang, K.Y. T2-weighted balanced steady-state free procession MRI evaluated for diagnosing placental adhesion disorder in late pregnancy. Eur. Radiol. 2018, 28, 3770–3778. [Google Scholar] [CrossRef] [PubMed]
- Safety Evaluation Dossier Supporting a Generally Recodnized as Safe (GRAS) Conclusion for Orange Pomace. Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm (accessed on 20 November 2019).
- Ietta, F.; Maioli, E.; Daveri, E.; Oliveira, J.G.; da Silva, R.J.; Romagnoli, R.; Cresti, L.; Avanzati, A.M.; Paulesu, L.; Barbosa, B.D.; et al. Rottlerin-mediated inhibition of toxoplasma gondii growth in BeWo trophoblast-like cells. Sci. Rep. 2017, 7, 1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clabault, H.; Flipo, D.; Guibourdenche, J.; Fournier, T.; Sanderson, J.T.; Vaillancourt, C. Effects of selective serotonin-reuptake inhibitors (SSRIs) on human villous trophoblasts syncytialization. Toxicol. Appl. Pharm. 2018, 349, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, R.; Rao, A.J. Trophoblast ‘pseudo-tumorigenesis’: Significance and contributory factors. Reprod. Biol. Endocrinol. 2004, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Bu, J.; Yu, L.; Chen, J.; Liu, H. Nobiletin improves propofol-induced neuroprotection via regulating Akt/mTOR and TLR 4/NF-κB signaling in ischemic brain injury in rats. Biomed. Pharmacother. 2017, 91, 494–503. [Google Scholar] [CrossRef]
- Yasuda, N.; Ishii, T.; Oyama, D.; Fukuta, T.; Agato, Y.; Sato, A.; Shimizu, K.; Asai, T.; Asakawa, T.; Kan, T.; et al. Neuroprotective effect of nobiletin on cerebral ischemia-reperfusion injury in transient middle cerebral artery-occluded rats. Brain Res. 2014, 1559, 46–54. [Google Scholar] [CrossRef]
- Liu, B.; Huang, J.; Zhang, B. Nobiletin protects against murine l-arginine-induced acute pancreatitis in association with downregulating p38MAPK and AKT. Biomed. Pharmacother. 2016, 81, 104–110. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.Y.; Wang, M.Q.; Zheng, J.K.; Gao, Z.L.; Xu, F.; Zhang, G.D.; Xiao, H. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol. Nutr. Food Res. 2015, 59, 2383–2394. [Google Scholar] [CrossRef] [Green Version]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007, 251, 168–178. [Google Scholar] [CrossRef]
- Ma, W.; Feng, S.; Yao, X.; Yuan, Z.; Liu, L.; Xie, Y. Nobiletin enhances the efficacy of chemotherapeutic agents in ABCB1 overexpression cancer cells. Sci. Rep. 2015, 5, 18789. [Google Scholar] [CrossRef]
- Delbridge, A.R.; Grabow, S.; Strasser, A.; Vaux, D.L. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer 2016, 16, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Radha, G.; Raghavan, S.C. BCL2: A promising cancer therapeutic target. BBA Rev. Cancer 2017, 1868, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Simpson, E.R.; Brown, K.A. p53: Protection against tumor growth beyond effects on cell cycle and apoptosis. Cancer Res. 2015, 75, 5001–5007. [Google Scholar] [CrossRef] [Green Version]
- Mrakovcic, M.; Kleinheinz, J.; Frohlich, L.F. p53 at the crossroads between different types of HDAC inhibitor-mediated cancer cell death. Int. J. Mol. Sci. 2019, 20, 2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M. Census and evaluation of p53 target genes. Oncogene 2017, 36, 3943–3956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |








| 12 h | 24 h | 36 h | 48 h | |
|---|---|---|---|---|
| NOB 10 μM | 21.22% ± 8.42% bA | 32.22% ± 6.67% aA | 19.44% ± 9.17% bA | 15.56% ± 8.46% bA |
| NOB 33 μM | 20.44% ± 7.17% bA | 30.77% ± 9.07% aA | 12.12% ± 7.80% cB | 17.34% ± 5.31% bcA |
| NOB 100 μM | 23.44% ± 4.20% aA | 15.33% ± 3.00% bB | 10.17% ± 2.93% cB | 9.17% ± 3.08% cB |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhang, R.; Liu, J.; Wang, H.; Wang, Z.; Liu, J.; Shan, Y.; Yu, H. The Effects of 5,6,7,8,3′,4′-Hexamethoxyflavone on Apoptosis of Cultured Human Choriocarcinoma Trophoblast Cells. Molecules 2020, 25, 946. https://doi.org/10.3390/molecules25040946
Zhang M, Zhang R, Liu J, Wang H, Wang Z, Liu J, Shan Y, Yu H. The Effects of 5,6,7,8,3′,4′-Hexamethoxyflavone on Apoptosis of Cultured Human Choriocarcinoma Trophoblast Cells. Molecules. 2020; 25(4):946. https://doi.org/10.3390/molecules25040946
Chicago/Turabian StyleZhang, Mengling, Rui Zhang, Jian Liu, Hongliang Wang, Zhen Wang, Juan Liu, Yang Shan, and Huanling Yu. 2020. "The Effects of 5,6,7,8,3′,4′-Hexamethoxyflavone on Apoptosis of Cultured Human Choriocarcinoma Trophoblast Cells" Molecules 25, no. 4: 946. https://doi.org/10.3390/molecules25040946