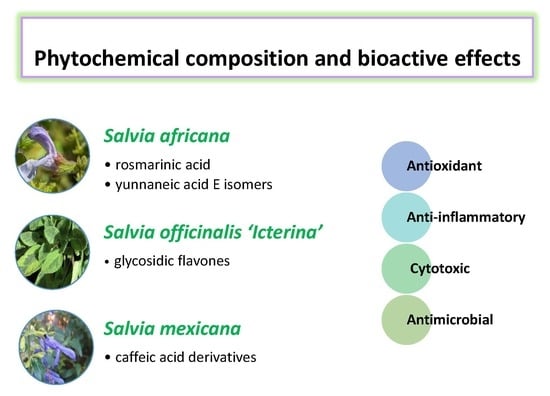
Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenolic Compounds in Salvia Aqueous Extracts
2.2. Bioactive Properties of Salvia Aqueous Extracts
2.2.1. Antibacterial Activity
2.2.2. Antioxidant Activity
2.2.3. Anti-Inflammatory Activity
2.2.4. Cytotoxic Activity
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Extraction of Phenolic Compounds
3.4. Identification and Quantification of Phenolic Compounds
3.5. Antibacterial Activity
3.6. Antioxidant Activity
3.6.1. 2,2-Diphenyl-1-Picrylhydrazyl Radical (DPPH•) Scavenging Test
3.6.2. Reducing Power Test
3.6.3. Thiobarbituric Acid Reactive Substances (TBARS)
3.6.4. β-Carotene Bleaching Inhibition Assay
3.7. Anti-Inflammatory Activity
3.8. Cytotoxicity in Tumor Cell Lines and Primary Porcine Liver Cells
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karousou, R.; Hanlidou, E.; Kokkini, S. The Sage Plants of Greece: Distribution and Infraspecific Variation. In Sage, the Genus Salvia; Kintzios, S.E., Ed.; Taylor & Francis e-Library: Amsterdam, The Netherlands, 2005; pp. 27–46. ISBN 0-203-34348-4. [Google Scholar]
- Dweck, A.C. Introduction. The Folklore and Cosmetic Use of Various Salvia Species. In Sage, the Genus Salvia; Kintzios, S.E., Ed.; Taylor & Francis e-Library: Amsterdam, The Netherlands, 2005; pp. 1–25. ISBN 0-203-34348-4. [Google Scholar]
- Carović-Stanko, K.; Petek, M.; Grdiša, M.; Pintar, J.; Bedeković, D.; Ćustić, M.H.; Satovic, Z. Medicinal plants of the family lamiaceae as functional foods-A review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef]
- Ozkan, G.; Kamiloglu, S.; Ozdal, T.; Boyacioglu, D.; Capanoglu, E. Potential use of Turkish medicinal plants in the treatment of various diseases. Molecules 2016, 21, 257. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, M.; Jokic, S.; Molnar, M.; Jašic, M.; Babic, J.; Jukic, H.; Banjari, I. Salvia officinalis L. Preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Jassbi, A.R.; Zare, S.; Firuzi, O.; Xiao, J. Bioactive phytochemicals from shoots and roots of Salvia species. Phytochem. Rev. 2016, 15, 829–867. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.; Tofană, M.; Socaci, S.A.; Pop, C.; Rotar, A.M.; Salanţă, L. Determination of Antioxidant Capacity and Antimicrobial Activity of Selected Salvia Species. Bull. UASVM Food Sci. Technol. 2016, 73. [Google Scholar] [CrossRef]
- Arizmendi, M.; Constanza, M.; Lourdes, J.; Ivonne, F.; Edgar, L. Effect of nectar feeders over diversity and abundance of S. mexicana and S. fulgens in a sub-urban park next to Mexico City. Biol. Conserv. 2007, 136, 155–158. [Google Scholar] [CrossRef]
- Frontana-Uribe, B.A.; Escárcega-Bobadilla, M.V.; Estrada-Reyes, R.; Morales-Serna, J.A.; Salmón, M.; Cárdenas, J. A new languidulane diterpenoid from Salvia mexicana var. mexicana. Molecules 2011, 16, 8866–8873. [Google Scholar] [CrossRef]
- S. africana. Available online: http://pza.sanbi.org/salvia-africana (accessed on 13 July 2019).
- Kamatou, G.P.P.; Makunga, N.P.; Ramogola, W.P.N.; Viljoen, A.M. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Van Zyl, R.L.; Vuuren, S.F. Van Chemical Composition, Leaf Trichome Types and Biological Activities of the Essential Oils of Four Related Salvia Species Indigenous to Southern Africa. J. Essent. Oil Res. 2006, 18, 72–79. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M.; Steenkamp, P. Antioxidant, antiinflammatory activities and HPLC analysis of South African Salvia species. Food Chem. 2010, 119, 684–688. [Google Scholar] [CrossRef]
- Martino, L.; Roscigno, G.; Mancini, E.; Falco, E.; Feo, V. Chemical Composition and Antigerminative Activity of the Essential Oils from Five Salvia Species. Molecules 2010, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Van Zyl, R.L.; Van Vuuren, S.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Viljoen, A.M. Seasonal variation in essential oil composition, oil toxicity and the biological activity of solvent extracts of three South African Salvia species. South African J. Bot. 2008, 74, 230–237. [Google Scholar] [CrossRef]
- Pereira, R.; Catarino, M.D.; Afonso, A.F.; Silva, A.M.S.; Cardoso, S.M. Salvia elegans, Salvia greggii and Salvia officinalis Decoctions: Antioxidant Activities and Inhibition of Carbohydrate and Lipid Metabolic Enzymes. Molecules 2018, 23, 3169. [Google Scholar] [CrossRef]
- Alimpić, A.; Knežević, A.; Milutinović, M.; Stević, T.; Šavikin, K.; Stajić, M.; Marković, S.; Marin, P.D.; Matevski, V.; Duletić-Laušević, S. Biological activities and chemical composition of Salvia amplexicaulis Lam. extracts. Ind. Crops Prod. 2017, 105, 1–9. [Google Scholar] [CrossRef]
- Alimpić, A.; Pljevljakušić, D.; Šavikin, K.; Knežević, A.; Ćurčić, M.; Veličković, D.; Stević, T.; Petrović, G.; Matevski, V.; Vukojević, J.; et al. Composition and biological effects of Salvia ringens (Lamiaceae) essential oil and extracts. Ind. Crops Prod. 2015, 76, 702–709. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.S.F.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. The Health-Benefits and Phytochemical Profile of Salvia apiana and Salvia farinacea var. victoria blue Decoctions. Antioxidants 2019, 8. [Google Scholar]
- Toplan, C.G.G.; Kurkcuoglu, M.; Goger, F.; Iscan, G.; Agalar, H.G.; Mat, A.; Baser, K.H.C.; Koyuncu, M.; Sarıyar, G. Composition and biological activities of Salvia veneris Hedge growing in Cyprus. Ind. Crops Prod. 2017, 97, 41–48. [Google Scholar] [CrossRef]
- Ben Farhat, M.; Landoulsi, A.; Chaouch-Hamada, R.; Sotomayor, J.A.; Jordán, M.J. Characterization and quantification of phenolic compounds and antioxidant properties of Salvia species growing in different habitats. Ind. Crops Prod. 2013, 49, 904–914. [Google Scholar] [CrossRef]
- Kozics, K.; Klusová, V.; Srančíková, A.; Mučaji, P.; Slameňová, D.; Hunáková, Ľ.; Kusznierewicz, B.; Horváthová, E. Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 2013, 141, 2198–2206. [Google Scholar] [CrossRef]
- Kostic, M.; Petrovic, M.B.; Jevtovic, T.; Jovic, M.; Petrovic, A.; Slavoljub, Ž. Anti-inflammatory effect of the Salvia sclarea L. ethanolic extract on lipopolysaccharide-induced periodontitis in rats. J. Ethnopharmacol. J. 2017, 199, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Dinparast, L.; Zengin, G.; Sarikurkcu, C.; Bahadori, S.; Asghari, B.; Movahhedin, N. Functional components, antidiabetic, anti-Alzheimer’s disease, and antioxidant activities of Salvia syriaca L. Int. J. Food Prop. 2017, 20, 1761–1772. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Asghari, B.; Dinparast, L.; Zengin, G.; Sarikurkcu, C.; Abbas-Mohammadi, M.; Bahadori, S. Salvia nemorosa L.: A novel source of bioactive agents with functional connections. Food Sci. Thecnology 2017, 75, 42–50. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Münch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Joe, Y.; Zheng, M.; Kim, H.J.; Kim, S.; Uddin, M.J.; Park, C.; Ryu, D.G.; Kang, S.S.; Ryoo, S.; Ryter, S.W.; et al. Salvianolic acid B exerts vasoprotective effects through the modulation of heme oxygenase-1 and arginase activities. J. Pharmacol. Exp. Ther. 2012, 341, 850–858. [Google Scholar] [CrossRef]
- Jang, H.H.; Cho, S.Y.; Kim, M.J.; Kim, J.B.; Lee, S.H.; Lee, M.Y.; Lee, Y.M. Anti - inflammatory effects of Salvia plebeia R. Br extract in vitro and in ovalbumin - induced mouse model. Biol. Res. 2016, 1–11. [Google Scholar] [CrossRef]
- Akkol, E.K.; Göger, F.; Koşar, M.; Başer, K.H.C. Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem. 2008, 108, 942–949. [Google Scholar] [CrossRef]
- Moharram, F.A.-E.; Marzouk, M.S.; El-Shenawy, S.M.; Gaara, A.H.; El Kady, W.M. Polyphenolic profile and biological activity of Salvia splendens leaves. J. Pharm. Pharmacol. 2012, 64, 1678–1687. [Google Scholar] [CrossRef]
- Boukhary, R.; Raafat, K.; Ghoneim, A.I.; Aboul-ela, M.; El-lakany, A. Anti-Inflammatory and Antioxidant Activities of Salvia fruticosa: An HPLC Determination of Phenolic Contents. Evidence-based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Ibrahim, T. a Chemical composition and biological activity of extracts from Salvia bicolor Desf. growing in Egypt. Molecules 2012, 17, 11315–11334. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, S.; Xia, H.; Lou, H.; Zhu, F.; Sun, L. Anti-inflammatory activities and potential mechanisms of phenolic acids isolated from Salvia miltiorrhiza f. alba roots in THP-1 macrophages. J. Ethnopharmacol. 2018, 222, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, L.; Rupasinghe, H.P.V. Antiproliferative effects of extracts from Salvia officinalis L. and Saliva miltiorrhiza Bunge on hepatocellular carcinoma cells. Biomed. Pharmacother. 2017, 85, 57–67. [Google Scholar] [CrossRef]
- Shahneh, F.Z.; Baradaran, B.; Orangi, M.; Zamani, F. In vitro Cytotoxic Activity of Four Plants Used in Persian Traditional Medicine. Adv. Farm. Bull. 2013, 3, 453–455. [Google Scholar]
- Ferreira, F.M.; Dinis, L.T.; Azedo, P.; Galhano, C.I.C.; Simões, A.; Cardoso, S.M.; Domingues, M.R.M.; Pereira, O.R.; Palmeira, C.M.; Peixoto, F.P. Antioxidant capacity and toxicological evaluation of Pterospartum tridentatum flower extracts. CyTA - J. Food 2012, 10, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Pereira, O.R.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Identification of phenolic constituents of Cytisus multiflorus. Food Chem. 2012, 131, 652–659. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Neto, R.T.; Silva, A.M.S.; Cardoso, S.M. Health-promoting effects of Thymus herba-barona, Thymus pseudolanuginosus, and Thymus caespititius decoctions. Int. J. Mol. Sci. 2017, 18, 1879. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Válega, M.; Silva, A.M.S.; Cardoso, S.M. Metabolites and biological activities of Thymus zygis, Thymus pulegioides, and Thymus fragrantissimus grown under organic cultivation. Molecules 2018, 23, 1514. [Google Scholar] [CrossRef] [Green Version]
- Shami, A.M.M.; Philip, K.; Muniandy, S. Synergy of antibacterial and antioxidant activities from crude extracts and peptides of selected plant mixture. BMC Complement. Altern. Med. 2013, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Saraiva, S.C.; Sobral, A.J.F.N.; Cardoso, S.M. Characterization of phenolic constituents and evaluation of antioxidant properties of leaves and stems of Eriocephalus africanus. Arab. J. Chem. 2018, 11, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, L.; Ferreira, M.J.; Queiro, B.; Ferreira, I.C.F.R.; Baptista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Souza, A.H.P.; Corrêa, R.C.G.; Barros, L.; Calhelha, R.C.; Santos-buelga, C.; Peralta, R.M.; Bracht, A.; Matsushita, M.; Ferreira, I.C.F.R. Phytochemicals and bioactive properties of Ilex paraguariensis: An in-vitro comparative study between the whole plant, leaves and stems. Food Res. Int. 2015, 78, 286–294. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| RT | UVmax | [M − H]− | MS 2 Main Fragments | Probable Compound | S. afr * | S. ict * | S. mex * |
|---|---|---|---|---|---|---|---|
| 1.5 | 275 | 149 | 103, 87, 131, 59 | 2,4-DimethylBA | 3.4 ± 0.2 | 4.7 ± 0.1 | 5.1 ± 0.1 |
| 1.7 | 205 | 191 | 111, 173 | Quinic Ac | D | 0.40 ± 0.05 | D |
| 3.6 | 280 | 197 | 179, 73, 153 | Danshensu | 6.5 ± 0.3 | D | 3.5 ± 0.1 |
| 4.2 | 278 | 315 | 153 | Protoc Ac Hex | - | - | D |
| 5.0 | 290 sh, 324 | 353 | 191, 179, 135 | cis-3-O-CQA | - | - | 3.8 ± 0.0 |
| 5.3 | 290, 327 | 311 | 149, 179 | Caftaric Ac | - | - | 3.50 ± 0.04 |
| 8.3 | 313 | 295 | 163 | p-Coum Ac Pent | - | - | 0.03 ± 0.01 |
| 8.8 | 290 sh, 325 | 353 | 191 | trans-5-O-CQA | - | - | 1.70 ± 0.06 |
| 9.4 | ND | 325 | 163, 119 | Caff Hex | - | - | 0.3 ± 0.0 |
| 9.5 | 290 sh, 325 | 353 | 173, 179, 191 | 4-O-CQA | - | - | 2.5 ± 0.3 |
| 9.7 | 290 sh, 323 | 179 | 135 | Caff Ac | 2.3 ± 0.1 | 2.50 ± 0.04 | 0.9 ± 0.1 |
| 11.7 | 271 | 1077 | 358, 179, 296, 494 | Galotannin Der | D | - | - |
| 12.1 | 271, 336 | 593 | 473, 503, 353 | Api-6-C-Glc-7-O-Glc | - | 3.5 ± 0.1 | - |
| 12.3 | 289, 329 | 295 | 207, 179, 133, 135 | Caff Malic Ac | - | - | 1.00 ± 0.03 |
| 12.6 | 286, 320 | 313 | 269, 179, 135 | SA F | - | 2.7 ± 0.2 | D |
| 13.1 | 291, 311 | 637 | 351, 285, 193 | Ferulic Ac Der | - | 0.6 ± 0.1 | - |
| 13.5 | 274 | 571 | 527, 483, 439, 373 | YA E (isomer 1) | 3.8 ± 0.3 | - | 4.4 ± 0.2 |
| 13.9 | 256, 267 sh, 345 | 447 | 327, 357 | Lut-C-Hex | - | - | 4.1 ± 0.2 |
| 13.9 | 281, 345 | 477 | 301, 373, 343, 397 | Hydroxy-Lut-GlcA | D | 2.90 ± 0.05 | - |
| 14.1 | 276 | 571 | 527, 439, 553, 483 | YA E (isomer 2) | 5.2 ± 0.7 | - | - |
| 14.3 | 276 | 597 | 579, 355, 312, 295, 197, 179 | YA F | 8.7 ± 0.9 | - | - |
| 14.7 | 274 | 571 | 527, 509, 553, 483, 285 | YA E (isomer 3) | 18.3 ± 0.9 | - | 2.80 ± 0.01 |
| 14.8 | 267, 345 | 621 | 351, 269 | Api-diGlcA | - | D | - |
| 15.1 | 276 | 555 | 313, 357 | SA K | 3.9 ± 0.3 | - | - |
| 15.4 | 274 | 571 | 527, 553, 509, 329 | YA E (isomer 4) | D | - | - |
| 15.6 | 235, 275, 320 | 539 | 297, 359, 495, 279 | YA D (isomer 1) | D | - | - |
| 15.9 | 280, 333 | 461 | 285 | Scut-O-GlcA | - | 9.7 ± 0.2 | - |
| 15.9 | 277 | 539 | 341, 253, 315, 359 | YA D (isomer 2) | 2.4 ± 0.2 | - | 3.5 ± 0.1 |
| 16.1 | 255, 266 sh, 345 | 461 | 285 | Lut-7-O-GlcA | 18.7 ± 1.2 | 18.2 ± 0.4 | - |
| 16.5 | 268 | 571 | 527, 409 | YA E (isomer 5) | 30.8 ± 1.7 | - | - |
| 17.2 | 278 | 717 | 519, 475, 537, 339 | SA B (isomer 1) | - | - | 1.50 ± 0.02 |
| 17.3 | 279 | 571 | 527, 553, 329 | YA E (isomer 6) | 15.4 ± 0.84 | - | - |
| 17.6 | 283 | 719 | 359, 539, 521, 341 | Sag Ac (isomer 1) | 6.0 ± 0.3 | 9.1 ± 0.4 | 1.20 ± 0.01 |
| 18.1 | 269, 329 | 431 | 269 | Api Hex | - | 1.70 ± 0.03 | - |
| 18.3 | 238, 341 | 607 | 299, 284 | Chrys Rut | - | - | D |
| 18.4 | 267, 337 | 445 | 269, 175 | Api-O-GlcA | 4.6 ± 0.2 | 32.8 ± 0.5 | - |
| 18.6 | 270 | 717 | 555, 519, 475, 357 | SA B (isomer 2) | 3.2 ± 0.2 | 3.3 ± 0.2 | - |
| 18.7 | 284, 330 sh | 609 | 301 | Hesperidin | - | - | 0.50 ± 0.03 |
| 19.0 | 290 sh, 328 | 359 | 161, 179, 197, 223 | RA | 77.0 ± 3.6 | 52.7 ± 0.5 | 29.4 ± 0.6 |
| 19.2 | 282 sh, 327 | 537 | 493, 295 | Caff RA (isomer 1) | 3.0 ± 0.2 | - | - |
| 19.5 | 285 sh, 305 | 537 | 493, 295 | Caff RA (isomer 2) | 14.2 ± 1.5 | - | 1.0 ± 0.1 |
| 19.8 | 278 | 719 | 521, 341, 359 | Sag Ac (isomer 2) | - | - | 2.2 ± 0.1 |
| 21.4 | 285sh, 330 | 537 | 456, 493, 375, 359 | Caff RA (isomer 3) | 2.5 ± 1.0 | - | - |
| Sum | 231.6 ± 7.5 | 144.1 ± 2.7 | 72.8 ± 0.7 | ||||
| Total Phenolic Content 1 | 350.6 ± 14.9 | 229.0 ± 44.0 | 158.9 ± 38.0 | ||||
| Bacteria | S. africana | S. officinalis ‘Icterina’ | S. mexicana | Nisin |
|---|---|---|---|---|
| Staphylococcus aureus | 0.63/1.25 | 0.94/0.94 | 1.19/1.19 | <0.63/<0.63 |
| Staphylococcus epidermidis | 1.25/1.25 | 3.75/3.75 | 4.75/9.50 | <0.63/<0.63 |
| Salmonella typhimurium | 5.0/5.0 | 3.75/3.75 | >9.50/>9.50 | 0.50/0.50 |
| Escherichia coli | 10.0/10.0 | 7.5/7.5 | 9.50/9.50 | 0.50/1.0 |
| Pseudomonas aeruginosa | >10.0/>10.0 | 7.5/7.5 | 9.50/9.50 | 1.0/1.0 |
| Assays | S. africana | S. officinalis ‘Icterina’ | S. mexicana | Standard |
|---|---|---|---|---|
| Antioxidant Activity (EC50, μg/mL) | ||||
| DPPH• | 6.6 ± 0.7 b | 10.4 ± 0.2 a | 10.0 ± 1.1 a | 6.68 ± 0.7 b |
| Ferric reducing power | 21.2 ± 2.7 b | 42.3 ± 3.1 a | 34.0 ± 6.5 a | 16.1 ± 2.0 b |
| TBARS inhibition | 21.0 ± 0.3 c | 23.0 ± 0.2 b | 26.2 ± 0.9 a | 23.0 ± 1.0 b |
| β-Carotene bleaching inhibition | 128.6 ± 6.3 c | 146.6 ± 7.0 b | 164.6 ± 7.7 a | 41.7 ± 0.3 d |
| Anti-Inflammatory Activity (EC50, μg/mL) | ||||
| NO production inhibition | 47.8 ± 2.1 b | 60.3 ± 1.5 a | 66.3 ± 5.4 a | 16.0 ± 1.0 c |
| Cytotoxic activity (GI50, μg/mL) | ||||
| HepG2 (hepatocellular carcinoma) | 42.5 ± 4.2 a | 48.9 ± 4.4 a | 52.4 ± 4.9 a | 1.0 ± 0.2 b |
| HeLa (cervical carcinoma) | 58.8 ± 4.5 b | 89.2 ± 7.2 a | 61.0 ± 5.6 b | 2.0 ± 0.1 c |
| MCF-7 (breast carcinoma) | 61.3 ± 9.8 a | 71.0 ± 3.4 a | 66.2 ± 4.6 a | 1.0 ± 0.04 b |
| NCI-H460 (non-small cell lung cancer) | 286.6 ± 17.6 a | 273.3 ± 14.3 a | 257.6 ± 21.4 a | 1.0 ± 0.1 b |
| PLP2 (non-tumour cells) | 336.4 ± 10.8 a | 304.9 ± 11.1 a | 296.8 ± 7.3 a | 3.0 ± 1.0 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts. Molecules 2019, 24, 4327. https://doi.org/10.3390/molecules24234327
Afonso AF, Pereira OR, Fernandes Â, Calhelha RC, Silva AMS, Ferreira ICFR, Cardoso SM. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts. Molecules. 2019; 24(23):4327. https://doi.org/10.3390/molecules24234327
Chicago/Turabian StyleAfonso, Andrea F., Olívia R. Pereira, Ângela Fernandes, Ricardo C. Calhelha, Artur M. S. Silva, Isabel C.F.R. Ferreira, and Susana M. Cardoso. 2019. "Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts" Molecules 24, no. 23: 4327. https://doi.org/10.3390/molecules24234327