Base-Promoted Chemodivergent Formation of 1,4-Benzoxazepin-5(4H)-ones and 1,3-Benzoxazin-4(4H)-ones Switched by Solvents
Abstract
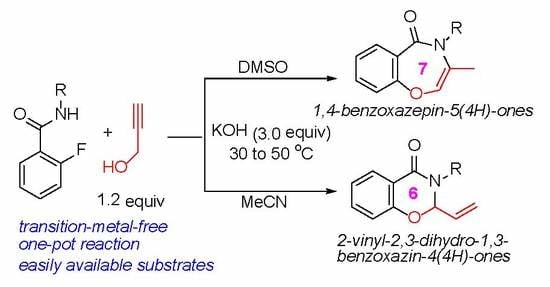
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Typical Experimental Procedure for the Synthesis of N-Propyl-3-methyl-1,4-benzoxazepin-5(4H)-one (2a)
3.3. Characterization Data of Products:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Wei, Y.; Shi, M. Divergent synthesis of carbo- and heterocycles via gold-catalyzed reactions. ACS Catal. 2016, 6, 2515–2524. [Google Scholar] [CrossRef]
- Zhan, G.; Du, W.; Chen, Y.-C. Switchable divergent asymmetric synthesis via organocatalysis. Chem. Soc. Rev. 2017, 46, 1675–1692. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Z.; Zhang, X.; Jia, Y. Divergent strategy in natural product total synthesis. Chem. Rev. 2018, 118, 3752–3832. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Kumar, K.; Waldmann, H. Ligand-directed divergent synthesis of carbo- and heterocyclic ring systems. Angew. Chem. Int. Ed. 2018, 57, 5212–5226. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Q.; Zhu, J. Metamorphosis of cycloalkenes for the divergent total synthesis of polycyclic indole alkaloids. Chem. Soc. Rev. 2018, 47, 7882–7898. [Google Scholar] [CrossRef]
- Ding, D.; Mou, T.; Xue, J.; Jiang, X. Access to divergent benzo-heterocycles via a catalyst-dependent strategy in the controllable cyclization of o-alkynyl-N-methoxyl-benzamides. Chem. Commun. 2017, 53, 5279–5282. [Google Scholar] [CrossRef]
- Thenarukandiyil, R.; Dutta, C.; Choudhury, J. Switching of reaction pathway from C-C rollover to C-N ring-extension annulations. Chem. Eur. J. 2017, 23, 15529–15533. [Google Scholar] [CrossRef]
- Gao, W.-C.; Liu, T.; Cheng, Y.-F.; Chang, H.-H.; Li, X.; Zhou, R.; Wei, W.-L.; Qiao, Y. AlCl3-catalyzed intramolecular cyclization of N-arylpropynamides with N-sulfanylsuccinimides: Divergent synthesis of 3-sulfenyl quinolin-2-ones and azaspiro [4,5]trienones. J. Org. Chem. 2017, 82, 13459–13467. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, G.; Yang, X.; Li, X. Rhodium(III)-catalyzed chemodivergent annulations between N-methoxybenzamides and sulfoxonium ylides via C–H activation. Chem. Commun. 2018, 54, 670–673. [Google Scholar] [CrossRef]
- Sun, J.; Bai, D.; Wang, P.; Wang, K.; Zheng, G.; Li, X. Chemodivergent oxidative annulation of benzamides and enynes via 1,4-Rhodium migration. Org. Lett. 2019, 21, 1789–1793. [Google Scholar] [CrossRef]
- Davies, D.L.; Ellul, C.E.; Macgregor, S.A.; McMullin, C.L.; Singh, K. Experimental and DFT studies explain solvent control of C−H activation and product selectivity in the Rh(III)-catalyzed formation of neutral and cationic heterocycles. J. Am. Chem. Soc. 2015, 137, 9659–9669. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Q.; Qian, C.; Liu, C.; Liu, W.; Chen, K.; Lei, A. Solvent-enabled radical selectivities: Controlled syntheses of sulfoxides and sulfides. Angew. Chem. Int. Ed. 2016, 55, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Xiao, Y.; Zhao, S.; Hu, X.-Q.; Xu, P.-F. Catalyst-free chemoselective synthesis of 3,4-dihydroquinazoline-2-thiones and 2-imino[1,3]benzothiazines. J. Org. Chem. 2016, 81, 10499–10505. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, Z.; Li, J.; Wu, W.; Liu, H.; Jiang, H. Palladium-catalyzed cross-coupling of alkynyl carboxylic acids with isocyanides: Solvent-controlled selective synthesis of 5-iminofuranones and 5-iminopyrrolones. Adv. Synth. Catal. 2017, 359, 3509–3514. [Google Scholar] [CrossRef]
- Jia, J.; Yu, A.; Ma, S.; Zhang, Y.; Li, K.; Meng, X. Solvent-controlled switchable domino reactions of MBH carbonate: Synthesis of benzothiophene fused α-pyran, 2,3-dihydrooxepine and oxatricyclodecene derivatives. Org. Lett. 2017, 19, 6084–6087. [Google Scholar] [CrossRef]
- Peng, J.-B.; Wu, X.-F. Ligand- and solvent-controlled regio- and chemodivergent carbonylative reactions. Angew. Chem. Int. Ed. 2018, 57, 1152–1160. [Google Scholar] [CrossRef]
- Guo, S.; Wang, F.; Tao, L.; Zhang, X.; Fan, X. Solvent-dependent copper-catalyzed indolyl C3-oxygenation and N1-cyclization reactions: Selective synthesis of 3H-indol-3-ones and indolo[1,2-c]quinazolines. J. Org. Chem. 2018, 83, 3889–3896. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Wang, Y.-Q.; Huang, L.-T.; Zhu, L.-Q.; Feng, Y.-Y.; Lu, Y.-M.; Zhao, Q.-Y.; Wang, X.-Q.; Wang, Z. NaI-mediated divergent synthesis of isatins and isoindigoes: A new protocol enabled by an oxidation relay strategy. Chem. Commun. 2018, 54, 8265–8268. [Google Scholar] [CrossRef]
- Wu, F.-P.; Peng, J.-B.; Qi, X.; Ying, J.; Wu, X.-F. Palladium-catalyzed solvent-dependent divergent synthesis of benzylformamides. Adv. Synth. Catal. 2018, 360, 3412–3417. [Google Scholar] [CrossRef]
- Yi, W.; Chen, W.; Liu, F.-X.; Zhong, Y.; Wu, D.; Zhou, Z.; Gao, H. Rh(III)-catalyzed and solvent-controlled chemoselective synthesis of chalcone and benzofuran frameworks via synergistic dual directing groups enabled regioselective C−H functionalization: A combined experimental and computational study. ACS Catal. 2018, 8, 9508–9519. [Google Scholar] [CrossRef]
- Xu, L.; Chen, J.; Chu, L. Solvent-tuned chemoselective carboazidation and diazidation of alkenes via iron catalysis. Org. Chem. Front. 2019, 6, 512–516. [Google Scholar] [CrossRef]
- Kamei, K.; Maeda, N.; Ogino, R.; Koyama, M.; Nakajima, M.; Tatsuoka, T.; Ohno, T.; Inoue, T. New 5-HT1A receptor agonists possessing 1,4-benzoxazepine scaffold exhibit highly potent anti-ischemic effects. Bioorg. Med. Chem. Lett. 2001, 11, 595–598. [Google Scholar] [CrossRef]
- Kamei, K.; Maeda, N.; Nomura, K.; Shibata, M.; Katsuragi-Ogino, R.; Koyama, M.; Nakajima, M.; Inoue, T.; Ohno, T.; Tatsuoka, T. Synthesis, SAR studies, and evaluation of 1,4-benzoxazepine derivatives as selective 5-HT1A receptor agonists with neuroprotective effect: Discovery of Piclozotan. Bioorg. Med. Chem. 2006, 14, 1978–1992. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-Q.; Song, M.-X.; Wei, C.-X.; Sun, Z.-G.; Quan, Z.-S. Synthesis and evaluation of 7-substituted-3,4-dihydrobenzo[f]- [1,4]oxazepin-5(2H)-ones as anticonvulsant and hypnotic agents. Med. Chem. Res. 2011, 20, 996–1004. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Shao, P.-L.; Zhao, Y. Catalytic divergent synthesis of 3H or 1H pyrroles by [3 + 2] cyclization of allenoates with activated isocyanides. J. Am. Chem. Soc. 2015, 137, 628–631. [Google Scholar] [CrossRef]
- Feng, J.-J.; Lin, T.-Y.; Zhu, C.-Z.; Wang, H.; Wu, H.-H.; Zhang, J. The divergent synthesis of nitrogen heterocycles by rhodium(I)-catalyzed intermolecular cycloadditions of vinyl aziridines and alkynes. J. Am. Chem. Soc. 2016, 138, 2178–2181. [Google Scholar] [CrossRef]
- Naganathan, S.; Andersen, D.L.; Andersen, N.G.; Lau, S.; Lohse, A.; Sørensen, M.D. Process development and scale-up of a benzoxazepine-containing kinase inhibitor. Org. Process Res. Dev. 2015, 19, 721–734. [Google Scholar] [CrossRef]
- Popp, T.A.; Tallant, C.; Rogers, C.; Fedorov, O.; Brennan, P.E.; Müller, S.; Knapp, S.; Bracher, F. Development of selective CBP/P300 benzoxazepine bromodomain inhibitors. J. Med. Chem. 2016, 59, 8889–8912. [Google Scholar] [CrossRef]
- Cheng, Q.-Q.; Lankelma, M.; Wherritt, D.; Arman, H.; Doyle, M.P. Divergent rhodium-catalyzed cyclization reactions of enoldiazoacetamides with nitrosoarenes. J. Am. Chem. Soc. 2017, 139, 9839–9842. [Google Scholar] [CrossRef]
- Monzani, M.V.; Coltro, G.; Sala, A.; Sardina, M. Pharmacokinetics of ITF 296 (Sinitrodil) a novel organic nitrate, in healthy volunteers. Eur. J. Pharmaceut. Sci. 1999, 7, 179–184. [Google Scholar] [CrossRef]
- Minghetti, P.; Casiraghi, A.; Montanari, L.; Monzani, M.V. In vitro skin permeation of Sinitrodil, a member of a new class of nitrovasodilator drugs. Eur. J. Pharmaceut. Sci. 1999, 7, 231–236. [Google Scholar] [CrossRef]
- Madhavan, G.R.; Chakrabarti, R.; Reddy, K.A.; Rajesh, B.M.; Balraju, V.; Rao, P.B.; Rajagopalan, R.; Iqbal, J. Dual PPAR-αand –γ activators derived from novel benzoxazinone containing thiazolidinediones having antidiabetic and hypolipidemic potential. Bioorg. Med. Chem. 2006, 14, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, G.; Alper, H. Domino ring-opening/carboxamidation reactions of N-tosyl aziridines and 2-halophenols/pyridinol: Efficient synthesis of 1,4-benzo- and pyrido-oxazepinones. Org. Lett. 2010, 12, 192–195. [Google Scholar] [CrossRef]
- Pandey, S.; Kumar, S.V.; Kant, R.; Chauhan, P.M.S. Base mediated 7-exo-dig intramolecular cyclization of Ugi–propargyl precursors: A highly efficient and regioselective synthetic approach toward diverse 1,4-benzoxazepine-5(2H)-ones. Org. Biomol. Chem. 2014, 12, 5346–5350. [Google Scholar] [CrossRef]
- Meiresonne, T.; Verniest, G.; Kimpe, N.D.; Mangelinckx, S. Synthesis of 2-fluoro-1,4-benzoxazines and 2-fluoro-1,4-benzoxazepin-5-ones by exploring the nucleophilic vinylic substitution (SNV) reaction of gem-difluoroenamides. J. Org. Chem. 2015, 80, 5111–5124. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, Q.; Lu, L.; Ma, Y.; Auyoung, G.H.L.; Hua, R. Base-promoted nucleophilic fluoroarenes substitution of C-F bonds. Tetrahedron 2018, 74, 303–307. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Mehmood, H.; Lv, J.; Hua, R. Base-promoted SNAr reactions of fluoro- and chloroarenes as a route to N-aryl indoles and carbazoles. Molecules 2019, 24, 1145. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Lu, L.; Mehmood, H.; Khan, D.M.; Hua, R. Quinazolinone synthesis through base-promoted SNAr reaction of ortho-fluorobenzamides with amides followed by cyclization. ACS Omega 2019, 4, 8207–8213. [Google Scholar] [CrossRef]
- Huang, Q.; Hua, R. Rhodium(I)-catalyzed reductive cyclocarbonylation of internal alkynes: Atom- economic process for synthesis of 2-cyclopenten-1-ones, 5-alkylidenefuran- 2(5H)-ones and indan-1-ones. Chem. Eur. J. 2009, 15, 3817–3822. [Google Scholar] [CrossRef]
- Li, J.; Hua, R. Stereodivergent ruthenium-catalyzed transfer semihydrogenation of diaryl alkynes. Chem. Eur. J. 2011, 17, 8462–8465. [Google Scholar] [CrossRef]
- Nizami, T.A.; Hua, R. Silver-catalyzed chemoselective annulation of propargyl amines with alkynes for access to pyridines and pyrroles. Tetrahedron 2017, 73, 6080–6084. [Google Scholar] [CrossRef]
- Nizami, T.A.; Hua, R. Cycloaddition of 1,3-butadiynes: Efficient synthesis of carbo- and heterocycles. Molecules 2014, 19, 13788–13802. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Nizami, T.A. Synthesis of heterocycles by using propargyl compounds as versatile synthons. Mini-Rev. Org. Chem. 2018, 15, 198–207. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, R. C–H activation and alkyne annulation via automatic or intrinsic directing groups: Towards high step economy. Chem. Rec. 2018, 18, 556–569. [Google Scholar] [CrossRef]
- One substrate example of the cyclization of 2-(2-propynyl)oxy-benzamide under the different base and solvent conditions to afford the corresponding either 3-methyl-1,4-Benzoxazepin-5(4H) -one (34%) or 2-vinyl-1,3-Benzoxazin-4(4H)-one (34%) with low chemoselectivities and low yields was reported, see: Scherrer, V.; Jackson-Mülly, M.; Zsindely, J.; Schmid, H. Base catalysed cyclizations of 2-(2-propynyl)oxy-benzamide systems. Helv. Chim. Acta 1978, 61, 716–731. [Google Scholar] [CrossRef]
- Both structures can be confirmed based on their 1H- and 13C-NMR. 2g and 3g are also further confirmed by their X-ray diffraction studies (CCDC1908830 (2g); CCDC1908829 (3g)).
- Trofimov, B.A.; Schmidt, E.Y. Acetylenes in the superbase-promoted assembly of carbocycles and heterocycles. Acc. Chem. Res. 2018, 51, 1117–1130. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Qiu, R.; Wang, X.; Long, J.; Zhu, L.; Au, C.-T.; Xu, X. Cesium hydroxide-catalyzed isomerization of terminal alkynes for the synthesis of O-allenes and N-allenes. Tetrahedron Lett. 2015, 56, 5504–5507. [Google Scholar] [CrossRef]
- As required by one of the reviewers, we have done the D-labeling study by using KOD. Unfortunately, we cannot observe the obvious kinetic isotope effect (KIE), and the experimental results are reported in Supporting Information.
- Seth, K.; Nautiyal, M.; Purohit, P.; Parikh, N.; Chakraborti, A.K. Palladium catalyzed Csp2–H activation for direct aryl hydroxylation: The unprecedented role of 1,4-dioxane as a source of hydroxyl radicals. Chem. Commun. 2015, 51, 191–194. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2 and 3 are not available from the authors. |







| Entry | Base (equivalent) | Solvent | Temperature (°C) /Time (h) | Yield (%) b |
|---|---|---|---|---|
| 1 | KOH (3) | DMSO | 50/12 | 45% (2a) + 12% (3a) |
| 2 | KOH (3) | DMSO | 30/12 | 52% (2a) + 7% (3a) |
| 3 | KOH (3) | DMSO | 30/12 + 50/12 | 54% (2a) |
| 4 c | KOH (3) | DMSO | 30/12 + 50/12 | 45% (2a) |
| 5 d | KOH (3) | DMSO | 30/12 + 50/12 | 47% (2a) |
| 6 | KOH (3) | DMSO | 30/12 + 70/12 | 41% (2a) |
| 7 | KOH (1) | DMSO | 30/12 + 50/12 | 44% (2a) |
| 8 | KOH (2) | DMSO | 30/12 + 50/12 | 47% (2a) |
| 9 | NaOH (3) | DMSO | 30/12 + 50/12 | 33% (2a) |
| 10 | K2CO3 (3) | DMSO | 30/12 + 50/12 | ~ 10% (2a) |
| 11 | Cs2CO3 (3) | DMSO | 30/12 + 50/12 | comlex mixture |
| 12 | t-BuOK (3) | DMSO | 30/12 + 50/12 | complex mixture |
| 13 e | KOH (3) | DMAc | 30/12 + 50/12 | 52% (2a) + 26% (3a) |
| 14 | KOH (3) | 1,4-dioxane | 30/12 + 50/12 | 35% (2a) + ~10% (3a) |
| 15 | KOH (3) | THF | 30/12 + 50/12 | ~10% (2a) + 48% (3a) |
| 16 | KOH (3) | DMF | 30/12 + 50/12 | ~10% (2a) + 48% (3a) |
| 17 | KOH (3) | MeCN | 30/12 + 50/12 | trace (2a) + 83% (3a) |
| 18 | KOH (3) | MeCN | 30/12 + 50/12 | 12% (2a) + 53% (3a) |
| 19d | KOH (3) | DMSO/MeCN (1:1 in volume) | 30/12 + 50/12 | trace (2a) + 79% (3a) |
| 20 | KOH (3) | MeCN | 30/12 + 70/12 | trace (2a) + 62% (3a) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Wang, Y.; Hua, R. Base-Promoted Chemodivergent Formation of 1,4-Benzoxazepin-5(4H)-ones and 1,3-Benzoxazin-4(4H)-ones Switched by Solvents. Molecules 2019, 24, 3773. https://doi.org/10.3390/molecules24203773
Chen Q, Wang Y, Hua R. Base-Promoted Chemodivergent Formation of 1,4-Benzoxazepin-5(4H)-ones and 1,3-Benzoxazin-4(4H)-ones Switched by Solvents. Molecules. 2019; 24(20):3773. https://doi.org/10.3390/molecules24203773
Chicago/Turabian StyleChen, Qian, Yunpeng Wang, and Ruimao Hua. 2019. "Base-Promoted Chemodivergent Formation of 1,4-Benzoxazepin-5(4H)-ones and 1,3-Benzoxazin-4(4H)-ones Switched by Solvents" Molecules 24, no. 20: 3773. https://doi.org/10.3390/molecules24203773