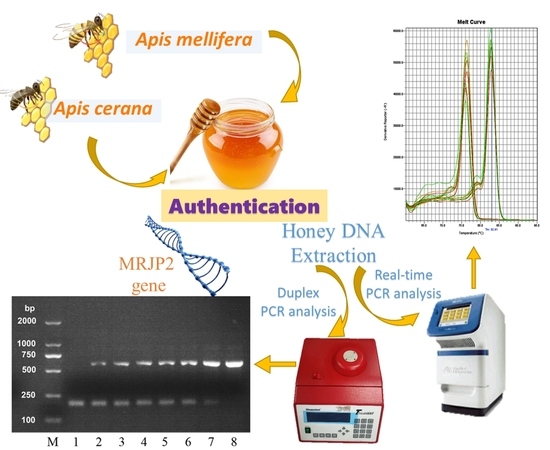
Authentication of Apis cerana Honey and Apis mellifera Honey Based on Major Royal Jelly Protein 2 Gene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of PCR Primers
2.2. Specific Detection of MRJP2 Gene in Honey Using PCR
2.3. Detection Sensitivity and Application in Adulteration
2.4. Real-Time PCR Method Development and Melt Curve Analysis
3. Materials and Methods
3.1. Honey and Honeybee Samples
3.2. DNA Extraction
3.3. Design and Selection of PCR Primers
3.4. PCR
3.5. Real-Time PCR and Melt Curve Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aries, E.; Burton, J.; Carrasco, L.; De Rudder, O.; Maquet, A. Scientific Support to the Implementation of a Coordinated Control Plan with a View to Establishing the Prevalence of Fraudulent Practices in the Marketing of Honey. NSANTE/2015; JRC Technical Report 2016, JRC104749. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/oc_control-progs_honey_jrc-tech-report_2016.pdf (accessed on 14 December 2018).
- Wu, L.; Du, B.; Vander Heyden, Y.; Chen, L.; Zhao, L.; Wang, M.; Xue, X. Recent advancements in detecting sugar-based adulterants in honey–A challenge. TrAC Trends Anal. Chem. 2017, 86, 25–38. [Google Scholar] [CrossRef]
- Guler, A.; Kocaokutgen, H.; Garipoglu, A.V.; Onder, H.; Ekinci, D.; Biyik, S. Detection of adulterated honey produced by honeybee (Apis mellifera L.) colonies fed with different levels of commercial industrial sugar (C3 and C4 plants) syrups by the carbon isotope ratio analysis. Food Chem. 2014, 155, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Ropciuc, S.; Paduret, S.; Todosi, E. Rheological analysis of honeydew honey adulterated with glucose, fructose, inverted sugar, hydrolysed inulin syrup and malt wort. LWT 2018, 95, 1–8. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S.; Paduret, S. Honey Adulteration Detection Using Raman Spectroscopy. Food Anal. Methods 2018, 11, 959–968. [Google Scholar] [CrossRef]
- Arias, M.C.; Sheppard, W.S. Phylogenetic relationships of honey bees (Hymenoptera: Apinae: Apini) inferred from nuclear and mitochondrial DNA sequence data. Mol. Phylogen. Evol. 2005, 37, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Grazina, L.; Mafra, I.; Costa, J.; Pinto, M.A.; Duc, H.P.; Oliveira, M.B.P.; Amaral, J.S. Novel diagnostic tools for Asian (Apis cerana) and European (Apis mellifera) honey authentication. Food Res. Int. 2018, 105, 686–693. [Google Scholar] [CrossRef]
- Partap, L.; Verma, L. Asian bees and beekeeping: Issues and initiatives. In Proceedings of the 4th Asian Apiculture Association International Conference, Kathmandu, Nepal, 23–28 March 1998; pp. 3–14. [Google Scholar]
- Verma, L. Beekeeping in Integrated Mountain Development; Oxford & IBH Publ.: New Delhi, India, 1991; p. 237. [Google Scholar]
- Won, S.-R.; Li, C.-Y.; Kim, J.-W.; Rhee, H.-I. Immunological characterization of honey major protein and its application. Food Chem. 2009, 113, 1334–1338. [Google Scholar] [CrossRef]
- He, X.; Wang, W.; Qin, Q.; Zeng, Z.; Zhang, S.; Barron, A.B. Assessment of flight activity and homing ability in Asian and European honey bee species, Apis cerana and Apis mellifera, measured with radio frequency tags. Apidologie 2013, 44, 38–51. [Google Scholar] [CrossRef]
- Lee, D.-C.; Lee, S.-Y.; Cha, S.-H.; Choi, Y.-S.; Rhee, H.-I. Discrimination of native bee-honey and foreign bee-honey by SDS-PAGE. Korean J. Food Sci. Technol. 1998, 30, 1–5. [Google Scholar]
- Won, S.-R.; Lee, D.-C.; Ko, S.H.; Kim, J.-W.; Rhee, H.-I. Honey major protein characterization and its application to adulteration detection. Food Res. Int. 2008, 41, 952–956. [Google Scholar] [CrossRef]
- Ramón-Sierra, J.M.; Ruiz-Ruiz, J.C.; de la Luz Ortiz-Vázquez, E. Electrophoresis characterisation of protein as a method to establish the entomological origin of stingless bee honeys. Food Chem. 2015, 183, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Chen, Y.-F.; Wu, Y.-Q.; Si, J.-J.; Zhang, C.-P.; Zheng, H.-Q.; Hu, F.-L. Discrimination of the entomological origin of honey according to the secretions of the bee (Apis cerana or Apis mellifera). Food Res. Int. 2018, in press. [Google Scholar] [CrossRef]
- Zuccato, V.; Finotello, C.; Menegazzo, I.; Peccolo, G.; Schievano, E. Entomological authentication of stingless bee honey by 1H NMR-based metabolomics approach. Food Control 2017, 82, 145–153. [Google Scholar] [CrossRef]
- Amaral, J.; Meira, L.; Oliveira, M.; Mafra, I. Advances in authenticity testing for meat speciation. In Advances in Food Authenticity Testing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 369–414. [Google Scholar]
- Prosser, S.W.; Hebert, P.D. Rapid identification of the botanical and entomological sources of honey using DNA metabarcoding. Food Chem. 2017, 214, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-K.; Lee, D.-C.; Choi, S.-H. Detection of Korean Native Honey and European Honey by Using Duplex Polymerase Chain Reaction and Immunochromatographic Assay. Korean J. Food Sci. Anim. Resour. 2017, 37, 599. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-W.; Liu, G.-H.; Dong, X.; Lin, R.-Q.; Song, H.-Q.; Huang, S.-Y.; Yuan, Z.-G.; Zhao, G.-H.; Zhu, X.-Q. The complete mitochondrial genome of the Asiatic cavity-nesting honeybee Apis cerana (Hymenoptera: Apidae). PLoS ONE 2011, 6, e23008. [Google Scholar] [CrossRef] [PubMed]
- Salvato, P.; Simonato, M.; Battisti, A.; Negrisolo, E. The complete mitochondrial genome of the bag-shelter moth Ochrogaster lunifer (Lepidoptera, Notodontidae). BMC Genom. 2008, 9, 331. [Google Scholar] [CrossRef]
- Cook, C.E. The complete mitochondrial genome of the stomatopod crustacean Squilla mantis. BMC Genom. 2005, 6, 105. [Google Scholar] [CrossRef]
- Bottero, M.T.; Dalmasso, A. Animal species identification in food products: Evolution of biomolecular methods. Vet. J. 2011, 190, 34–38. [Google Scholar] [CrossRef]
- Fernandes, T.J.; Costa, J.; Oliveira, M.B.P.; Mafra, I. DNA barcoding coupled to HRM analysis as a new and simple tool for the authentication of Gadidae fish species. Food Chem. 2017, 230, 49–57. [Google Scholar] [CrossRef]
- Utzeri, V.J.; Ribani, A.; Fontanesi, L. Authentication of honey based on a DNA method to differentiate Apis mellifera subspecies: Application to Sicilian honey bee (A. m. siciliana) and Iberian honey bee (A. m. iberiensis) honeys. Food Control 2018, 91, 294–301. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |




| Honey | Primer | DNA Sequences | Product Size (bp) |
|---|---|---|---|
| A. cerana | C-F | TTTAACAATAAAAATAATCAGAAGA | 212 |
| C-R | TTACATCCTAATTGATTTTAATGCG | ||
| A. mellifera | M-F | GCCATCCCTTGAAATTGTCACTCGT | 560 |
| M-R | TCTGCAAACGACCAATCAGGATAT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-Z.; Wang, S.; Chen, Y.-F.; Wu, Y.-Q.; Tian, J.; Si, J.-J.; Zhang, C.-P.; Zheng, H.-Q.; Hu, F.-L. Authentication of Apis cerana Honey and Apis mellifera Honey Based on Major Royal Jelly Protein 2 Gene. Molecules 2019, 24, 289. https://doi.org/10.3390/molecules24020289
Zhang Y-Z, Wang S, Chen Y-F, Wu Y-Q, Tian J, Si J-J, Zhang C-P, Zheng H-Q, Hu F-L. Authentication of Apis cerana Honey and Apis mellifera Honey Based on Major Royal Jelly Protein 2 Gene. Molecules. 2019; 24(2):289. https://doi.org/10.3390/molecules24020289
Chicago/Turabian StyleZhang, Yan-Zheng, Shuai Wang, Yi-Fan Chen, Yu-Qi Wu, Jing Tian, Juan-Juan Si, Cui-Ping Zhang, Huo-Qing Zheng, and Fu-Liang Hu. 2019. "Authentication of Apis cerana Honey and Apis mellifera Honey Based on Major Royal Jelly Protein 2 Gene" Molecules 24, no. 2: 289. https://doi.org/10.3390/molecules24020289