The Relationship between Autism Spectrum Disorder and Melatonin during Fetal Development
Abstract
:1. Introduction
2. Melatonin and Its Regulatory Effects on Circadian Rhythm
2.1. Melatonin and Its Putative Role in Regulating Fetal Circadian Rhythm
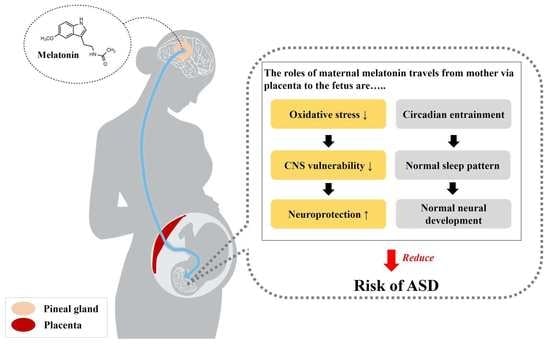
2.2. Regulatory Role of Melatonin in Fetal Development and Neuroprotection
3. Melatonin and Its Implications for Autism Spectrum Disorder (ASD)
3.1. Overview of ASD
3.2. Abnormal Melatonin Secretion and Its Implication in ASD
4. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vanecek, J. Cellular mechanisms of melatonin action. Physiol. Rev. 1998, 78, 687–721. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Sbonkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Parvez, S. Role of melatonin in traumatic brain injury and spinal cord injury. Sci. World J. 2014, 2014, 586270. [Google Scholar] [CrossRef] [PubMed]
- Sugden, D. Psychopharmacological effects of melatonin in mouse and rat. J. Pharmacol. Exp. Ther. 1983, 227, 587–591. [Google Scholar] [PubMed]
- Cardinali, D.P.; Srinivasan, V.; Brzezinski, A.; Brown, G.M. Melatonin and its analogs in insomnia and depression. J. Pineal Res. 2012, 52, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Mociková, K.; Mníchová, M.; Kubatka, P.; Bojková, B.; Ahlers, I.; Ahlersová, E. Mammary carcinogenesis induced in Wistar:Han rats by the combination of ionizing radiation and dimethylbenz(a)anthracene: Prevention with melatonin. Neoplasma 2000, 47, 227–229. [Google Scholar]
- Marková, M.; Adámeková, E.; Kubatka, P.; Bojková, B.; Ahlersová, E.; Ahlers, I. Effect of prolonged melatonin administration on metabolic parameters and organ weights in young male and female Sprague-Dawley rats. Acta Vet. Brno 2003, 72, 163–173. [Google Scholar] [CrossRef]
- Bilici, D.; Akpinar, E.; Kiziltunc, A. Protective effect of melatonin in carrageenan-induced acute local inflammation. Pharmacol. Res. 2002, 46, 133–139. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, S.R.; Spence, D.W.; Moscovitch, A.; Trakht, I.; Brown, G.M.; Cardinali, D.P. Potential use of melatonergic drugs in analgesia: Mechanisms of action. Brain Res. Bull. 2010, 81, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Sanchez-Barcelo, E.; Mediavilla, M.D.; Reiter, R.J. Significance of high levels of endogenous melatonin in mammalian cerebrospinal fluid and in the central nervous system. Curr. Neuropharmacol. 2010, 8, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Okamoto, K.; Hayashi, K.; Wakatsuki, A.; Tamura, S.; Sagara, Y. Maternal-fetal transfer of melatonin in pregnant women near term. J. Pineal Res. 1998, 25, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, S.E.; Zygouropoulos, N.; Zahiu, C.D.; Zagrean, A.M. Role of melatonin in embryo fetal development. J. Med. Life 2014, 7, 488–492. [Google Scholar] [PubMed]
- Zee, P.C.; Attarian, H.; Videnovic, A. Circadian rhythm abnormalities. Continuum (Minneap Minn) 2013, 19, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Berra, B.; Rizzo, A.M. Melatonin: Circadian rhythm regulator, chronobiotic, antioxidant and beyond. Clin. Dermatol. 2009, 27, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Nir, I.; Meir, D.; Zilber, N.; Knobler, H.; Hadjez, J.; Lerner, Y. Brief report: Circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. J. Autism Dev. Disord. 1995, 25, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Kulman, G.; Lissoni, P.; Rovelli, F.; Roselli, M.G.; Brivio, F.; Sequeri, P. Evidence of pineal endocrine hypofunction in autistic children. Neuro Endocrinol. Lett. 2000, 21, 31–34. [Google Scholar] [PubMed]
- Melke, J.; Goubran Botros, H.; Chaste, P.; Betancur, C.; Nygen, G.; Anckarsater, H.; Rastam, M.; Stahlberg, O.; Gillberg, I.C.; Delorme, R.; et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol. Psychiatry 2008, 13, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Barcelo, E.J.; Rueda, N.; Mediavilla, M.D.; Martinez-Cue, C.; Reiter, R.J. Clinical Uses of Melatonin in Neurological Diseases and Mental and Behavioural Disorders. Curr. Med. Chem. 2017, 24, 3851–3878. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Reiter, R.J.; Topal, T.; Manchester, L.C.; Oter, S.; Tan, D.X. Melatonin: An established antioxidant worthy of use in clinical trials. Mol. Med. 2009, 15, 43–50. [Google Scholar] [PubMed]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Benot, S.; Goberna, R.; Reiter, R.J.; Garcia-Maurino, S.; Osuna, C.; Guerrero, J.M. Physiological levels of melatonin contribute to the antioxidant capacity of human serum. J. Pineal Res. 1999, 27, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.; Hanes, M.A.; Farley, N.J. High physiological levels of melatonin in the bile of mammals. Life Sci. 1999, 65, 2523–2529. [Google Scholar] [CrossRef]
- Srinivasan, V. Melatonin oxidative stress and neurodegenerative diseases. Indian J. Exp. Biol. 2002, 40, 668–679. [Google Scholar] [PubMed]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores, L.J.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008, 25, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.E.; Alder, M.L.; Burgess, H.J.; Corbett, B.A.; Hundley, R.; Wofford, D.; Fawkes, D.B.; Wang, L.; Laudenslager, M.L.; Malow, B.A. Characterizing Sleep in Adolescents and Adults with Autism Spectrum Disorders. J. Autism Dev. Disord. 2017, 47, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.K.; Richdale, A.L.; Hazi, A.; Prendergast, L.A. Assessing the Dim Light Melatonin Onset in Adults with Autism Spectrum Disorder and No Comorbid Intellectual Disability. J. Autism Dev. Disord. 2017, 47, 2120–2137. [Google Scholar] [CrossRef] [PubMed]
- Souders, M.C.; Zavodny, S.; Eriksen, W.; Sinko, R.; Connell, J.; Kerns, C.; Schaaf, R.; Pinto-Martin, J. Sleep in Children with Autism Spectrum Disorder. Curr. Psychiatry Rep. 2017, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Geoffray, M.M.; Nicolas, A.; Speranza, M.; Georgieff, N. Are circadian rhythms new pathways to understand Autism Spectrum Disorder? J. Physiol. Paris 2016, 110, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, B.M.; Vaz, S.; Lee, E.A.L.; Thopmson, C.; Rogerson, J.M.; Falkmer, T. Effectiveness of Sleep-Based Interventions for Children with Autism Spectrum Disorder: A Meta-Synthesis. Pharmacotherapy 2017, 37, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Huguet, G.; Benabou, M.; Bourgeron, T. The Genetics of Autism Spectrum Disorders; Springer: Berlin, Germany, 2016; pp. 101–129. [Google Scholar]
- Pagan, C.; Delorme, R.; Callebert, J.; Goubran-Botros, H.; Amsellem, F.; Drouot, X.; Boudebesse, C.; Le Dudal, K.; Ngo-Nguyen, N.; Laouamri, H.; et al. The serotonin-N-acetylserotonin–melatonin pathway as a biomarker for autism spectrum disorders. Transl. Psychiatry 2014, 4, 479. [Google Scholar] [CrossRef] [PubMed]
- Pagan, C.; Goubran-Botros, H.; Delrome, R.; Benabou, M.; Lemiere, N.; Murray, K.; Amsellem, F.; Callebert, J.; Chaste, P.; Jamain, S.; et al. Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci. Rep. 2017, 7, 2096. [Google Scholar] [CrossRef] [PubMed]
- Benabou, M.; Rolland, T.; Leblond, C.S.; Millot, G.A.; Huguet, G.; Delorme, R.; Leboyer, M.; Pagan, C.; Callebert, J.; Maronde, E.; et al. Heritability of the melatonin synthesis variability in autism spectrum disorders. Sci. Rep. 2017, 7, 17746. [Google Scholar] [CrossRef] [PubMed]
- Braam, W.; Ehrhart, F.; Evelo, C.T.; Mass, A.P.; Smits, M.G.; Curfs, L. Low parental melatonin levels increases autism spectrum disorder risk in children. bioRxiv. 2016. [Google Scholar] [CrossRef]
- Won, J.; Jin, Y.; Choi, J.; Park, S.; Lee, T.H.; Lee, S.R.; Chang, K.T.; Hong, Y. Melatonin as a Novel Interventional Candidate for Fragile X Syndrome with Autism Spectrum Disorder in Humans. Int. J. Mol. Sci. 2017, 18, 1314. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W. Circadian rhythms. Horm. Res. 1998, 49, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Dugovic, C.; Zee, P.C. Current understanding of the circadian clock and the clinical implications for neurological disorders. Arch. Neurol. 2001, 58, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Haus, E.; Smolensky, M. Biological clocks and shift work: Circadian dysregulation and potential long-term effects. Cancer Causes Control 2006, 17, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Videnovic, A.; Lazar, A.S.; Barker, R.A.; Overeem, S. ‘The clocks that time us’[mdash] circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol. 2014, 10, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Takahashi, J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006, 15, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Bersten, D.C.; Sullivan, A.E.; Peet, D.J.; Whitelaw, M.L. bHLH-PAS proteins in cancer. Nat. Rev. Cancer 2013, 13, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007, 8, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gillette, M.U.; McArthur, A.J. Circadian actions of melatonin at the suprachiasmatic nucleus. Behav. Brain Res. 1996, 73, 135–139. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Smirnov, A.N. Nuclear melatonin receptors. Biochemistry (Mosc) 2001, 66, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Hayashi, T.; Yu, S.; Tajiri, N.; Bae, E.C.; Solomita, M.A.; Chheda, S.H.; Weinbren, N.L.; Parolini, O.; Borlongan, C.V. Human amniotic epithelial cells express melatonin receptor MT1, but not melatonin receptor MT2: A new perspective to neuroprotection. J. Pineal Res. 2011, 50, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; William, C.D. Principles and Practice of Sleep Medicine, 5th ed.; Elsevier Saunders: St. Louis, MO, USA; 2005; pp. 13–23. [Google Scholar]
- Morrissey, M.J.; Duntley, S.P.; Anch, A.M.; Nonneman, R. Active sleep and its role in the prevention of apoptosis in the developing brain. Med. Hypotheses 2004, 62, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, M.; Maas, Y.G.; Ariagno, R.L. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med. Rev. 2003, 7, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Andersen, I.M.; Kaczmarska, J.; McGrew, S.G.; Malow, B.A. Melatonin for insomnia in children with autism spectrum disorders. J. Child Neurol. 2008, 23, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Welin, A.K.; Svedin, P.; Lapatto, R.; Sultan, B.; Hagberg, H.; Gressens, P.; Kjellmer, I.; Mallard, C. Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr. Res. 2007, 61, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, I. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: Reduction by melatonin. J. Pineal Res. 2001, 31, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Serón-Ferré, M.; Torres-Farfan, C.; Forcelledo, M.L.; Valenzuela, G.J. The development of circadian rhythms in the fetus and neonate. Semin. Perinatol. 2001, 25, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Tain, Y.L.; Sheen, J.M.; Huang, L.T. Melatonin utility in neonates and children. J. Formos. Med. Assoc. 2012, 111, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.W.; Youngstrom, E.A.; Speer, L.; Embacher, R.; Law, P.; Constantino, J.; Findling, R.L.; Hardan, A.Y.; Eng, C. Validation of proposed DSM-5 criteria for autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 2011, 51, 28–40. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, Y.; Tesoriero, J.; Hogan, M.V.; Wieraszko, A. Melatonin regulates neuronal plasticity in the hippocampus. J. Neurosci. Res. 2003, 72, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Von Gall, C.; Garabette, M.L.; Kell, C.A.; Frenzel, S.; Dehghani, F.; Schumm-Draeger, P.M.; Weaver, D.R.; Korf, H.W.; Hastings, M.H.; Stehle, J.H. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat. Neurosci. 2002, 5, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Anderson, G.M.; Pichard, N.; Charbuy, H.; Touitou, Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol. Psychiatry 2005, 57, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Gringras, P.; Nir, T.; Breddy, J.; Frydman-Marom, A.; Findling, R.L. Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children with Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Biran, V.; Phan Duy, A.; Decobert, F.; Bednarek, N.; Alberti, C.; Baud, O. Is melatonin ready to be used in preterm infants as a neuroprotectant? Dev. Med. Child Neurol. 2014, 56, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Carloni, S.; Favrais, G.; Saliba, E.; Albertini, M.C.; Chalon, S.; Longini, M.; Gressens, P.; Buonocore, G.; Balduini, W. Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR-34a/silent information regulator 1 pathway. J. Pineal Res. 2016, 61, 370–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagrillo-Fagundes, L.; Assuncao Salustiano, E.M.; Yen, P.W.; Soliman, A.; Vaillancourt, C. Melatonin in pregnancy: Effects on brain development and CNS programming disorders. Curr. Pharm. Des. 2016, 22, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, T.E.; Wilkins, R.; Sherman, G.J. Birth weight percentiles by gestational age in Canada. Obstet. Gyecol. 1993, 81, 39–48. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Choi, J.; Won, J.; Hong, Y. The Relationship between Autism Spectrum Disorder and Melatonin during Fetal Development. Molecules 2018, 23, 198. https://doi.org/10.3390/molecules23010198
Jin Y, Choi J, Won J, Hong Y. The Relationship between Autism Spectrum Disorder and Melatonin during Fetal Development. Molecules. 2018; 23(1):198. https://doi.org/10.3390/molecules23010198
Chicago/Turabian StyleJin, Yunho, Jeonghyun Choi, Jinyoung Won, and Yonggeun Hong. 2018. "The Relationship between Autism Spectrum Disorder and Melatonin during Fetal Development" Molecules 23, no. 1: 198. https://doi.org/10.3390/molecules23010198