Isolation of Terpenoids from the Stem of Ficus aurantiaca Griff and their Effects on Reactive Oxygen Species Production and Chemotactic Activity of Neutrophils
Abstract
:1. Introduction
2. Results and Discussion
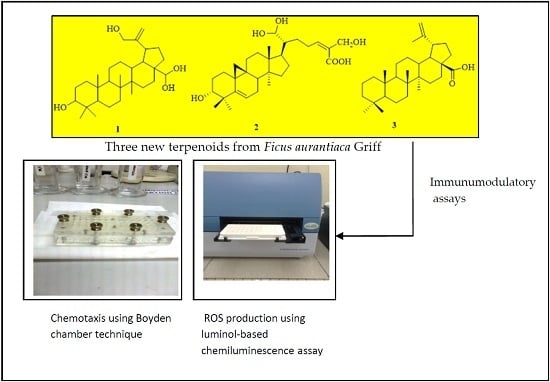
2.1. Characterization of the Isolated Compounds

| Positions | Compound 1 | Compound 2 | Compound 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 38.1 | 1.28 (d, 4H, J = 6.6) | 29.8 | 0.94, (m, 2H) | 36.1 | 1.41 (m, 1H) |
| 1.31 (m,1H) | ||||||
| 2 | 27.3 | 1.68 (d, 4H, J = 3.0) | 25.7 | 1.01 (m, 2H) | 28.2 | 1.42 (m, 1H) |
| 1.32 (m, 1H) | ||||||
| 3 | 80.1 | 3.39 (dd, 1H, J = 10.8, 4.8) | 80.1 | 3.44 (1H, t, J = 18.0), 4.68 (OH) | 36.7 | 1.42 (m, 1H) |
| 1.33 (m, 1H) | ||||||
| 4 | 39.6 | - | 43.0 | - | 30.8 | - |
| 5 | 55.6 | 1.32 (d, 1H, J = 7.2) | 151.0 | - | 59.6 | 1.34 (m, 1H) |
| 6 | 17.7 | 1.28 (m, 4H, J = 6.6) | 109.3 | 4.79 (t, 1H, J = 6.0) | 18.6 | 1.65 (m, 2H) |
| 7 | 34.1 | 1.28 (m, 4H) | 31.9 | 1.77 (m, 2H) | 37.4 | 1.46 (m, 1H) |
| 8 | 40.9 | - | 50.3 | 1.48 (m, 1H) | 40.1 | - |
| 9 | 50.3 | 0.88 (m, 10H) | 48.2 | - | 51.9 | 1.56 (m, 1H) |
| 10 | 37.3 | - | 39.9 | - | 36.7 | - |
| 11 | 21.0 | 1.61 (m, 4H) | 23.7 | 1.34 (m, 2H) | 20.6 | 1.54 (m, 1H) |
| 1.29 (m, 1H) | ||||||
| 12 | 25.1 | 1.68 (m, 4H) | 22.7 | 1.31 (m, 2H) | 25.2 | 1.55 (m, 1H) |
| 1.29 (m, 1H) | ||||||
| 13 | 37.9 | 1.34 (m, 1H) | 42.8 | - | 37.7 | 1.24 (m, 1H) |
| 14 | 42.8 | - | 40.9 | - | 42.9 | - |
| 15 | 27.4 | 1.26 (m, 4H) | 34.5 | 1.57 (m, 2H) | 39.7 | 1.49 (m, 1H) |
| 1.00 (m, 1H) | ||||||
| 16 | 35.5 | 1.26 (m, 4H) | 29.4 | 1.63 (m, 2H) | 40.4 | 1.86 (m, 1H) |
| 1.25 (m,1H) | ||||||
| 17 | 43.0 | - | 55.6 | 1.48 (m,1H) | 59.6 | - |
| 18 | 47.9 | 2.04 (m, 1H) | 27.4 | 1.03 (s, 2H) | 46.5 | 1.68 (m, 1H) |
| 19 | 48.2 | 2.38 (d, 1H, J = 7.2) | 17.7 | 1.04 s, 1H | 37.2 | 2.09 (m, 1H) |
| 20 | 150.9 | - | 35.3 | 1.68 (m, 1H) | 150.9 | - |
| 21 | 29.7 | 1.88–1.94 (m, 2H) | 95.1 | 4.80 (m, 1H) | 29.2 | 1.60 (m, 6H) |
| 22 | 39.9 | 1.34 (m, 4H) | 35.5 | 1.18 (m, 2H) | 23.0 | 1.75 (t, 2H, J = 5.4) |
| 23 | 14.5 | 0.79 (s, 3H) | 25.0 | 1.35 (m, 2H) | 22.1 | 0.83 (s, 6H) |
| 24 | 18.0 | 0.83 (s, 3H) | 150.9 | 4.57 (m, 1H) | 16.6 | 0.90 (s, 3H) |
| 25 | 15.9 | 0.94 (s, 3H) | 109.3 | - | 15.4 | 0.76 (s, 3H) |
| 26 | 16.1 | 1.01(s, 3H) | 69.2 | 4.03 (d, 1H, J = 12) | 17.6 | 0.89 (s, 3H) |
| 27 | 14.1 | 1.07 (s, 3H) | 173.8 | - | 13.9 | 0.73 (s, 3H) |
| 28 | 95.1 | 4.79 (t, 1H, J = 6 Hz) | 14.1 | 0.80 (s, 3H) | 183.5 | - |
| 29 | 109.3 | 4.69 (br s, 1H) | 15.9 | 0.85 (s, 1H) | 116.3 | 5.32 (brs, 1H) |
| 4.57 (br s, 1H) | ||||||
| 30 | 69.2 | 5.14 (m, 1H, H-30) | 16.5 | 0.92 (q, 3H) | 20.1 | 1.10 (s, 3H) |
| 5.19 (m, 1H, H-30) | ||||||

2.2. Chemotactic Activity

| Compounds | IC50 Value (μM) | ||
|---|---|---|---|
| ROS | Chemotaxis | ||
| PMNs | WB | ||
| 28,28,30-Trihydroxylupeol (1) | 9.2 ± 0.5 | 11.5 ± 0.05 | 6.8 ± 0.1 |
| 3,21,21,26-Tetrahydroxylanostanoic acid (2) | 0.9 ± 0.03 | 0.1 ± 0.3 | 2.8 ± 0.1 |
| Dehydroxybetulinic acid (3) | 0.9 ± 0.1 | 1.7 ± 0.1 | 2.5 ± 0.1 |
| Taraxerone (4) | 1.3 ± 0.1 | 4.6 ± 0.08 | 4.1 ± 0.5 |
| Taraxerol (5) | - | - | - |
| Ethyl palmitate (6) | 1.1 ± 0.05 | 0.7 ± 0.05 | 3.7 ± 0.2 |
| Herniarin (7) | 0.5 ± 0.5 | 1.6 ± 0.1 | 8.2 ± 0.2 |
| Stigmasterol (8) | - | - | - |
| Ursolic acid (9) | 0.8 ± 0.5 | 1.3 ± 0.8 | 3.6 ± 0.2 |
| Acetylursolic acid (10) | - | - | - |
| Aspirin (positive control) | 9.4 ± 0.5 | 11.6 ± 0.3 | - |
| Ibuprofen (positive control) | - | - | 6.7 ± 0.5 |
2.3. Reactive Oxygen Species (ROS) Inhibitory Activity of Isolated Pure Compounds on Human Whole Blood (WB) and PMNs

3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Chemicals, Reagents and Equipment
3.5. Isolation of Polymorphonnuclear Leucocytes (PMNs)
3.6. Cell Viability
3.7. Chemiluminescence Assay
3.8. Chemotaxis Assay
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vander, N.J.M.; Klerx, J.P.; Vandijk, H.; de Silva, K.T.; Labadie, R.P. Immunomodulatory activity of an aqueous extract of Azadirachta indica stem bark. J. Ethnopharmacol. 1987, 19, 125–131. [Google Scholar]
- Diwanay, S.; Chitre, D.; Patwardhan, B. Immunoprotection by botanical drugs in cancer chemotherapy. J. Ethnopharmacol. 2004, 90, 49–55. [Google Scholar]
- Gautam, M.; Diwanay, S.; Gairola, S.; Shinde, Y.; Patki, P.; Patwardhan, B. Immuno adjuvantpotential of Asparagus racemosus aqueous extract in experimental system. J. Ethnopharmacol. 2004, 91, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Jayathirtha, M.G.; Mishra, S.H. Preliminary immunomodulatory activities of methanol extracts of Eclipta alba and Centella asiatica. Phytomedicine 2004, 11, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Diasio, R.B.; LoBuglio, A.F. Immunomodulators: Immunosuppressive agents and immunostimulants. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 9th ed.; Hardman, J.G., Limbird, L.E., Molinoff, P.B., Ruddon, R.W., Eds.; McGraw-Hill: New York, NY, USA, 1996; pp. 1291–1307. [Google Scholar]
- Fordin, D.G. History and concepts of big plant genera. Taxon 2004, 53, 753–776. [Google Scholar] [CrossRef]
- Ronsted, N. Phylogeny, biogeography and ecology of Ficus section Malvanther (Moraceae). Mol. Phylogenet. Evol. 2008, 48, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.P.; Paavilainen, H.M.; Pawlus, A.D.; Newman, R.A. Ficus spp. (fig): Ethnobotany and potential as anticancer and anti-inflammatory agents. J. Ethnopharmacol. 2008, 119, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Simo, C.C.F.; Simeon, F.K.; Poumale, H.M.P.; Simo, I.K.; Ngadjui, B.T.; Green, I.R.; Krohn, K. Benjaminamide: A new ceramide and other compounds from the twigs of Ficus benjamin (Moraceae). Biochem. Syst. Ecol. 2008, 36, 238–243. [Google Scholar] [CrossRef]
- Li, R.W.; Leach, D.N.; Myers, S.P.; Lin, G.D.; Leach, G.J.; Waterman, P.G. A new anti-inflammatory glucoside from Ficus racemosa L. Planta. Med. 2004, 70, 421–426. [Google Scholar] [PubMed]
- Mandal, S.C.; Maity, T.K.; Das, J.; Das, B.P.; Pal, M. Anti-inflammatory evaluation of Ficus racemosa Linn. Leaf extract. J. Ethnopharmacol. 2000, 72, 87–92. [Google Scholar] [CrossRef]
- Sackyfio, A.C.; Lugeleka, O.M. The anti-inflammatory effect of a crude aqueous extract of the root bark of Ficus elastica in the rat. Arch. Int. Pharmacodyn. Ther. 1986, 281, 169–176. [Google Scholar]
- Shukla, R.; Gupta, S.; Gambhir, J.K.; Prabhu, K.M.; Murthy, P.S. Antioxidant effect of aqueous extract of the bark of Ficus benghalensis in hyper cholesterolaemic rabbits. J. Ethnopharmacol. 2004, 92, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Burkill, I.H. A Dictioanary of the Economic Products of the Malays Panisular; Ministry of Agriculture and Co-Operatives: Kuala Lumpur, Malaysia, 1966. [Google Scholar]
- Mawa, S.; Husain, K.; Jantan, I. Ficus carica L. (Moraceae): Phytochemistry, traditional uses and biological activities. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kamaruddin, M.S.; Latif, A. Tumbuhan Ubatan Malaysia Selangor; Pusat Pengurusan Penyelidikan: Bangi, Malaysia, 2002; Volume 1. [Google Scholar]
- Himanshu, J.; Arun, B.J.; Hemlata, S.; Gururaja, M.P.; Prajwal, R.S.; Subrahmanyam, A.V.S.; Satyanarayana, D. Fatty acids from Memecylonum bellatum (Burm). Asian J. Res. Chem. 2009, 2, 178–180. [Google Scholar]
- Ibrahim, A.; Najmuldeen, A.; Hamid, A.H.; Mohamad, K.; Khalijah, A.; Mehran, F.N.; Kamal, A.K.; Mat Ropi, M.; Hiroshi, M. Steroids from Chisocheton tomentosus. Malays. J. Sci. 2011, 30, 144–153. [Google Scholar]
- Jamal, A.K.; Yacoob, W.A.; Din, L.B. Triterpenes from the root bark of Phyllanthus columnaris. Aust. J. Basic Appl. Sci. 2009, 3, 1428–1431. [Google Scholar]
- Mahdi, A.; Amirhossein, S.; Mehrda, I. Synthesis and purification of 7-prenyloxycoumarins and herniarin as bioactive natural coumarins. Iran. J. Basic Med. Sci. 2009, 1, 63–69. [Google Scholar]
- Mawa, S.; Ikram, M.S. Chemical constituents of Garcinia prainiana. J. Sains Malays. 2012, 41, 585–590. [Google Scholar]
- Shahid, H.A.; Ali, M.; Kamran, J.N. New manglanostanoic acid from the stem bark of Megifera indica var. “Fazli”. J. Soudi Chem. Soc. 2014, 18, 561–565. [Google Scholar]
- Uddin, G.; Waliullah, M.; Siddiqui, B.S.; Alam, M.; Sadat, A.; Ahmad, A.; Uddin, A. Chemical constituents and phytotoxicity of solvent extracted fractions of stem bark of Grew optiva Drummond ex Burret. Middle East J. Sci. Res. 2011, 8, 85–91. [Google Scholar]
- Supaluk, P.; Puttirat, S.; Rungrot, C.; Somsak, R.; Virapong, P. New bioactive triterpenoids and antimalarial activity of Diospyros rubra lec. EXCLI J. 2010, 9, 1–10. [Google Scholar]
- Ahmed, W.; Khan, A.Q.; Malik, A. Two triterpenes from the leaves of Ficus carica. Planta Med. 1988, 54. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Kuo, Y.H. Novel triterpenoids from the arial parts of Ficus microcarpa. J. Org. Chem. 2002, 67, 7656–7661. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Shuaib, M.; Naquvi, K.J. New lanostane type triterpenes from the oleo-gum resin of Commiphora myrrha (NEES) ENGL. Int. J. Pharm. Pharm. Sci. 2014, 6, 372–375. [Google Scholar]
- Chiang, Y.M.; Chang, J.Y.; Kuo, C.C.; Kuo, Y.H.; Chang, C.Y. Cytotoxic triterpenes from the arial roots of Ficus microcarpa. Phytochemistry 2005, 66, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Spisani, S.; Vanzini, G.; Traniello, S. Inhibition of human leucocytes locomotion by anti-inflammatory drugs. Experientia 1979, 35, 803–804. [Google Scholar] [CrossRef] [PubMed]
- José-Luis, R. Effects of triterpenes on the immune system. J. Ethnopharmacol. 2010, 128, 1–14. [Google Scholar]
- Brinker, A.M.; Ma, J.; Lipsky, P.E.; Raskin, I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 2007, 68, 732–766. [Google Scholar] [CrossRef] [PubMed]
- Calis, I.; Yürüker, A.; demir, D.; Wright, A.D.; Sticher, O.; Luo, Y.D.; Pezzuto, J.M. Cycloartane triterpene glycosides from the roots of Astragalus melanophrurius. Planta Med. 1997, 63, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Behboudi, S.; Morein, B.; Villacres-Eriksson, M. In vitro activation of antigen presentin cells (APC) by defined composition of Quillaja saponaria molina triterpenoids. Clin. Exp. Immunol. 1996, 105, 26–30. [Google Scholar] [CrossRef] [PubMed]
- De las, H.B.; Rodríguez, B.; Boscá, L.; Villar, A.M. Terpenoids: Sources, structure elucidation and therapeutic potential in inflammation. Curr. Top. Med. Chem. 2003, 3, 53–67. [Google Scholar]
- Shashi, B.M.; Sucharita, S. Advances in triterpenoid research, 1990–1994. Phytochemistry 1997, 44, 1185–1236. [Google Scholar]
- Rukachaisirikul, V.; Ritthiwigrom, T.; Pinsa, A.; Sawangchote, P.; Taylor, W.C. Xanthones from the bark of Garcinia nigrolineata. Phytochemistry 2003, 64, 1149–1156. [Google Scholar] [CrossRef]
- Demirkiran, O.; Mesaik, M.A.; Beynek, H.; Abbaskhan, A.; Iqbal, C.M. Cellular reactive oxygen species inhibitory constituents of Hypericum thasium. Phytochemistry 2009, 70, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Koko, W.S.; Mesaik, M.A.; Yousof, S.; Galal, M.; Choudary, M.I. In vitro immonumodulating properties of selected Sudanese medicinal plants. J. Ethnopharmacol. 2008, 118, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Haklar, G.; Ozveri, E.S.; Yuksel, M.; Aktan, A.; Yalcin, A.S. Different kinds of reactive oxygen and nitrogen species were detected in colon and breast tumors. Cancer Lett. 2001, 165, 219–224. [Google Scholar] [CrossRef]
- Sacerdote, P.; Massi, P.; Panerai, A.E.; Parolaro, D. In vivo and in vitro treatment with the synthetic cannabinoid CP55,940 decreases the in vitro migration of macrophages in the rat: involvement of both CB1 and CB2 receptors. J. Neuroimmunol. 2000, 109, 155–163. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mawa, S.; Jantan, I.; Husain, K. Isolation of Terpenoids from the Stem of Ficus aurantiaca Griff and their Effects on Reactive Oxygen Species Production and Chemotactic Activity of Neutrophils. Molecules 2016, 21, 9. https://doi.org/10.3390/molecules21010009
Mawa S, Jantan I, Husain K. Isolation of Terpenoids from the Stem of Ficus aurantiaca Griff and their Effects on Reactive Oxygen Species Production and Chemotactic Activity of Neutrophils. Molecules. 2016; 21(1):9. https://doi.org/10.3390/molecules21010009
Chicago/Turabian StyleMawa, Shukranul, Ibrahim Jantan, and Khairana Husain. 2016. "Isolation of Terpenoids from the Stem of Ficus aurantiaca Griff and their Effects on Reactive Oxygen Species Production and Chemotactic Activity of Neutrophils" Molecules 21, no. 1: 9. https://doi.org/10.3390/molecules21010009