Triterpenoids from the Herbs of Salicornia bigelovii
Abstract
:1. Introduction
2. Results and Discussion


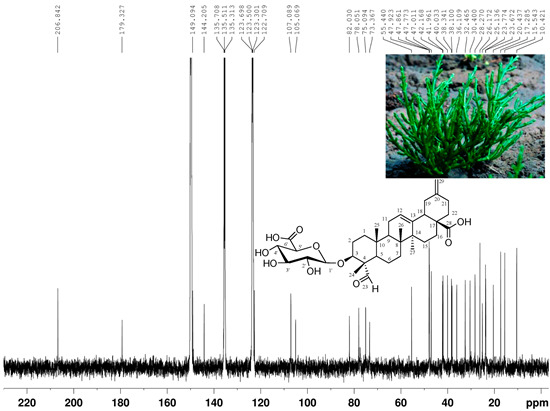
| No. | 1H-NMR [δ (ppm), mult., J (Hz)] | 13C-NMR [δ (ppm)] | DEPT | 1H-1HCOSY | HMBC |
|---|---|---|---|---|---|
| 1α | 0.88 (1H, m) | 38.1 (t) | CH2 | H-2 | |
| 1β | 1.39 (1H, m) | ||||
| 2α | 1.85 (1H, m) | 25.1 (t) | CH2 | H-3, H-1 | |
| 2β | 2.23 (1H, m) | ||||
| 3 | 4.17 (1H, dd, J = 4.5, 11.6) | 82.0 (d) | CH | H-2 | C-1, C-2 |
| 4 | 55.4 (s) | C | |||
| 5 | 1.31 (1H, m) | 47.7 (d) | CH | H-23 | C-4, C-10 |
| 6α | 1.35 (1H, m) | 20.4 (t) | CH2 | H-7 | |
| 6β | 1.01 (1H, m) | ||||
| 7α | 1.39 (1H, m) | 32.4 (t) | CH2 | H-6 | |
| 7β | 1.11 (1H, m) | ||||
| 8 | 40.0 (s) | C | |||
| 9 | 1.63 (1H, t, J = 7.7, 8.7) | 47.9 (d) | CH | ||
| 10 | 36.1 (s) | C | |||
| 11α | 1.86 (1H, m) | 23.6 (t) | CH2 | H-12 | |
| 11β | 1.86 (1H, m) | ||||
| 12 | 5.44 (1H, br s) | 122.7 (d) | CH | H-11 | C-9, C-14 |
| 13 | 144.2 (s) | C | |||
| 14 | 42.1 (s) | C | |||
| 15α | 1.13 (1H, m) | 28.2 (t) | CH2 | H-16 | |
| 15β | 2.10 (1H, m) | ||||
| 16α | 2.16 (1H, m) | 23.7 (t) | CH2 | H-15 | C-18 |
| 16β | 2.03 (1H, m) | ||||
| 17 | 47.0 (s) | C | |||
| 18 | 3.18 (1H, dd, J = 4.4) | 47.8 (d) | CH | H-19 | C-12, C-13 |
| 19α | 2.62 (1H, t, J = 13.7, 13.3) | 41.9 (t) | CH2 | H-18 | C-18, C-20, C-29 |
| 19β | 2.22 (1H, m) | ||||
| 20 | 149.0 (s) | C | |||
| 21α | 1.16 (1H, m) | 30.4 (t) | CH2 | H-22 | C-20 |
| 21β | 2.28 (1H, m) | ||||
| 22α | 1.87 (1H, m) | 38.3 (t) | CH2 | H-21 | C-20 |
| 22β | 2.18 (1H, m) | ||||
| 23 | 9.73 (1H, s) | 206.8 (s) | CHO | C-4, C-24 | |
| 24 | 1.27 (3H, s) | 10.4 (q) | CH3 | C-3, C-4, C-5, C-23 | |
| 25 | 0.77 (3H, s) | 15.5 (q) | CH3 | C-9 | |
| 26 | 0.89 (3H, s) | 17.2 (q) | CH3 | C-7, C-9 | |
| 27 | 1.24 (3H, s) | 26.1 (q) | CH3 | C-9, C-13, C-15 | |
| 28 | 179.3 (s) | C | |||
| 29α | 4.78 (1H, s) | 107.0 (t) | CH2 | C-19, C-21 | |
| 29β | 4.73 (1H, s) | ||||
| 1’ | 4.84 (1H, d, J = 7.8) | 105.0 (d) | CH | H-2’ | C-3 |
| 2’ | 3.88 (1H, m) | 75.0 (d) | CH | H-1’ | C-1’ |
| 3’ | 4.17 (1H, m) | 78.0 (d) | CH | H-4’, | C-4’, C-5’ |
| 4’ | 4.21 (1H, m) | 73.3 (d) | CH | H-3’, H-5’ | C-3’ |
| 5’ | 4.27 (1H, m) | 77.8 (d) | CH | H-4’ | C-4’ |
| 6’ | 179.3 (s) | C |
| Pathogenic Fungi | Inhibition after 72 h (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | AMB | |
| C. gloeosporioides | −2.53 | −5.24 | 27.61 | 24.15 | 13.65 | 14.77 | 33.27 | −1.90 | −1.48 | 7.54 | 69.21 | 73.89 |
| Pathogenic Fungi | 11 |
|---|---|
| A. alternata | 31.8 ± 1.3 |
| A. solani | 23.3 ± 1.5 |
| B. cinerea | 13.6 ± 0.6 |
| C. gloeosporioides | 34.0 ± 0.9 |
| F. graminearum | 28.8 ± 1.7 |
| F. verticilloides | 19.4 ± 1.1 |
| T. cucumeris | 13.9 ± 0.7 |
| S. sclerotiorum | 36.3 ± 2.1 |
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Fungus Strains and Positive Controls
3.5. Antifungal Assays in Vitro
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Q.Z.; Liu, X.F.; Shan, Y.; Guan, F.Q.; Chen, Y.; Wang, X.Y.; Wang, M.; Feng, X. Two new nortriterpenoid saponins from Salicornia bigelovii Torr. and their cytotoxic activity. Fitoterapia 2012, 83, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Glenn, E.P.; O’Leary, J.W.; Watson, M.C.; Thompson, T.L.; Kuehl, R.O. Salicornia bigelovii Torr.: An oilseed halophyte for seawater irrigation. Science 1991, 251, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.Q.; Shan, Y.; Zhao, Y.Y.; Wang, Q.Z.; Wang, M.; Sun, H.; Feng, X. Research development on pharmacological activities and mechanism of Salicornia plants. Chin. Pharm. Bull. 2013, 29, 1188–1191. [Google Scholar]
- Kim, J.Y.; Cho, J.Y.; Ma, Y.K.; Park, K.Y.; Lee, S.H.; Ham, K.S.; Lee, H.J.; Park, K.H.; Moon, J.H. Dicaffeoylquinic acid derivatives and flavonoid glucosides from glasswort (Salicornia herbacea L.) and their antioxidative activity. Food Chem. 2011, 125, 55–62. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Wang, H.; Liu, T.; Xin, Z. Two new noroleanane-type triterpene saponins from the methanol extract of Salicornia herbacea. Food Chem. 2014, 151, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Kong, C.S.; Lee, J.I.; Kim, H.; Park, H.Y.; Lee, H.S.; Lee, C.; Seo, Y. Evaluation of novel antioxidant triterpenoid saponins from the halophyte Salicornia herbacea. Bioorg. Med. Chem. Lett. 2012, 22, 4318–4322. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jadhav, A.N.; Khan, I.A. Triterpenoids from brazilian ginseng, Pfaffia paniculata. Planta Med. 2010, 76, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X.; Wang, M.; Jia, X.; Zhao, Y.; Dong, Y. Triterpene glycosides from Lonicera. II isolation and structural determination of glycosides from flower buds of Lonicera macranthoides. Chem. Nat. Compd. 2009, 45, 514–518. [Google Scholar] [CrossRef]
- Fang, J.B.; Yao, Z.; Chen, J.C.; Liu, Y.W.; Takaishi, Y.; Duan, H.Q. Cytotoxic triterpene saponins from Alternanthera philoxeroides. J. Asian Nat. Prod. Res. 2009, 11, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, H.; Wang, Q.H.; Yang, B.Y.; Kuang, H.X. A new feruloyl tyramine glycoside from the roots of Achyranthes bidentata. Chin. J. Nat. Med. 2012, 10, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Espada, A.; Rodriguez, J.; Villaverde, M.C.; Riguera, R. Hypoglucaemic triterpenoid saponins from Boussingaultia baselloides. Can. J.Chem. 1990, 68, 2039–2044. [Google Scholar] [CrossRef]
- Song, Q.Y.; Qi, W.Y.; Li, Z.M.; Zhao, J.; Chen, J.J.; Gao, K. Antifungal activities of triterpenoids from the roots of Astilbe myriantha Diels. Food Chem. 2011, 128, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–11 are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Y.; Li, H.; Guan, F.; Chen, Y.; Yin, M.; Wang, M.; Feng, X.; Wang, Q. Triterpenoids from the Herbs of Salicornia bigelovii. Molecules 2015, 20, 20334-20340. https://doi.org/10.3390/molecules201119695
Shan Y, Li H, Guan F, Chen Y, Yin M, Wang M, Feng X, Wang Q. Triterpenoids from the Herbs of Salicornia bigelovii. Molecules. 2015; 20(11):20334-20340. https://doi.org/10.3390/molecules201119695
Chicago/Turabian StyleShan, Yu, Huan Li, Fuqin Guan, Yu Chen, Min Yin, Ming Wang, Xu Feng, and Qizhi Wang. 2015. "Triterpenoids from the Herbs of Salicornia bigelovii" Molecules 20, no. 11: 20334-20340. https://doi.org/10.3390/molecules201119695