Sulfur Species, Bonding Environment, and Metal Mobilization in Mining-Impacted Lake Sediments: Column Experiments Replicating Seasonal Anoxia and Deposition of Algal Detritus
Abstract
:1. Introduction
2. Study Area, Materials, and Methods
2.1. Study Design
2.2. Sediment Core Collection
2.3. Algal Detritus
2.4. Sediment Profile Characterization
2.5. X-ray Spectroscopy
3. Results
3.1. Sediment Characteristics
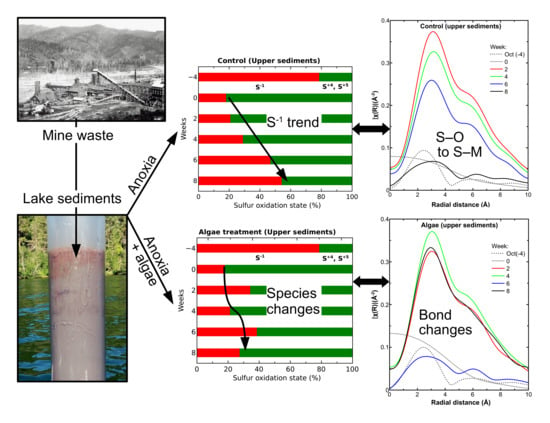
3.2. Sediment Sulfur Speciation and Bonding Environment Characteristics
3.3. Sediment Sulfur Speciation with Induced Anoxic Conditions
3.4. Upper Sediment Sulfur Bonding with Induced Anoxic Conditions
3.5. Redox-Sensitive Elements in Porewater
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bigham, J.M. Mineralogy of ochre deposits formed by sulfide oxidation. In Environmental Geochemistry of Sulfide Mine-Wastes; Jambor, J.L., Blowes, D.W., Eds.; Mineralogical Association of Canada: Quebec City, QC, Canada, 1994; Volume 22, pp. 103–132. ISBN 978-0-8412-2772-9. [Google Scholar]
- Bigham, J.M.; Nordstrom, D.K. Iron and aluminum hydroxysulfates from acid sulfate waters. Rev. Mineral. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Dudka, S.; Adriano, D.C. Environmental impacts of metal ore mining and processing: A review. J. Environ. Qual. 1997, 26, 590. [Google Scholar] [CrossRef]
- Moore, J.N.; Luoma, S.N. Hazardous wastes from large-scale metal extraction. A case study. Environ. Sci. Technol. 1990, 24, 1278–1285. [Google Scholar] [CrossRef]
- Pyatt, F.B.; Grattan, J.P. Some consequences of ancient mining activities on the health of ancient and modern human populations. J. Public Health 2001, 23, 235–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balistrieri, L.S.; Bookstrom, A.A.; Box, S.E.; Ikramuddin, M. Drainage from Adits and Tailings Piles in the Coeur d’Alene Mining District, Idaho; Sampling, Analytical Methods and Results; US Geological Survey Open-File Report; United States Geological Survey: Reston, VA, USA, 1998; p. 19.
- Horowitz, A.J.; Elrick, K.A.; Robbins, J.A.; Cook, R.B. A summary of the effects of mining and related activities on the sediment-trace element geochemistry of Lake Coeur d’Alene, Idaho, USA. J. Geochem. Explor. 1995, 52, 135–144. [Google Scholar] [CrossRef]
- Horowitz, A.J.; Elrick, K.A.; Robbins, J.A.; Cook, R.B. Effect of mining and related activities on the sediment trace element geochemistry of Lake Coeur d’Alene, Idaho, USA part II: Subsurface sediments. Hydrol. Process. 1995, 9, 35–54. [Google Scholar] [CrossRef]
- Paulson, A.J. Biogeochemical removal of Zn and Cd in the Coeur d’Alene River (Idaho, USA), downstream of a mining district. Sci. Total Environ. 2001, 278, 31–44. [Google Scholar] [CrossRef]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef]
- Haus, K.L.; Hooper, R.L.; Strumness, L.A.; Mahoney, J.B. Analysis of arsenic speciation in mine contaminated lacustrine sediment using selective sequential extraction, HR-ICPMS and TEM. Appl. Geochem. 2008, 23, 692–704. [Google Scholar] [CrossRef]
- Hochella, M.F., Jr.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363. [Google Scholar] [CrossRef] [Green Version]
- Hochella Jr, M.F.; Lower, S.K.; Maurice, P.A.; Penn, R.L.; Sahai, N.; Sparks, D.L.; Twining, B.S. Nanominerals, mineral nanoparticles, and Earth systems. Science 2008, 319, 1631–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, S.R.; Shafer, M.M.; Armstrong, D.E. Strong colloidal and dissolved organic ligands binding copper and zinc in rivers. Environ. Sci. Technol. 2007, 41, 6996–7002. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Schäfer, T. Metal retention and transport on colloidal particles in the environment. Elements 2005, 1, 205–210. [Google Scholar] [CrossRef]
- Langman, J.; Torso, K.; Moberly, J. Seasonal and basinal influences on the formation and transport of dissolved trace metal forms in a mining-impacted riverine environment. Hydrology 2018, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Langman, J.B.; Behrens, D.; Moberly, J.G. Seasonal formation and stability of dissolved metal particles in mining-impacted, lacustrine sediments. J. Contam. Hydrol. 2020, 232, 103655. [Google Scholar] [CrossRef] [PubMed]
- Plathe, K.L.; von der Kammer, F.; Hassellöv, M.; Moore, J.N.; Murayama, M.; Hofmann, T.; Hochella, M.F., Jr. The role of nanominerals and mineral nanoparticles in the transport of toxic trace metals: Field-flow fractionation and analytical TEM analyses after nanoparticle isolation and density separation. Geochim. Cosmochim. Acta 2013, 102, 213–225. [Google Scholar] [CrossRef]
- Wigginton, N.S.; Haus, K.L.; Jr, M.F.H. Aquatic environmental nanoparticles. J. Environ. Monit. 2007, 9, 1306–1316. [Google Scholar] [CrossRef]
- Wood, M.S.; Beckwith, M.A. Coeur d’Alene Lake, Idaho: Insights Gained From Limnological Studies of 1991–92 and 2004–06; United States Geological Survey: Reston, VA, USA, 2008; p. 40.
- La Force, M.J.; Hansel, C.M.; Fendorf, S. Arsenic speciation, seasonal transformations, and Co-distribution with iron in a mine waste-influenced palustrine emergent wetland. Environ. Sci. Technol. 2000, 34, 3937–3943. [Google Scholar] [CrossRef]
- Arora, B.; Şengör, S.S.; Spycher, N.F.; Steefel, C.I. A reactive transport benchmark on heavy metal cycling in lake sediments. Comput. Geosci. 2015, 19, 613–633. [Google Scholar] [CrossRef]
- Cummings, D.E.; March, A.W.; Bostick, B.; Spring, S.; Caccavo, F.; Fendorf, S.; Rosenzweig, R.F. Evidence for microbial Fe(III) reduction in anoxic, mining-impacted lake sediments (Lake Coeur d’Alene, Idaho). Appl. Environ. Microbiol. 2000, 66, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Morra, M.J.; Carter, M.M.; Rember, W.C.; Kaste, J.M. Reconstructing the history of mining and remediation in the Coeur d’Alene, Idaho Mining District using lake sediments. Chemosphere 2015, 134, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, J.S.; Topping, B.R.; Woods, P.F.; Carter, J.L. Free zinc ion and dissolved orthophosphate effects on phytoplankton from Coeur d’Alene Lake, Idaho. Environ. Sci. Technol. 2007, 41, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P. Harish Toxicity assessment of ZnO nanoparticles to freshwater microalgae Coelastrella terrestris. Environ. Sci. Pollut. Res. 2019, 26, 26991–27001. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, J.S.; Woods, P.F.; Berelson, W.M.; Balistrieri, L.S.; Carter, J.L.; Topping, B.R.; Fend, S.V. Importance of sediment–water interactions in Coeur d’Alene Lake, Idaho, USA: Management implications. Environ. Manag. 2003, 32, 348–359. [Google Scholar] [CrossRef]
- Woods, P.F.; Beckwith, M.A. Nutrient and Trace-Element Enrichment of COEUR d’Alene Lake, Idaho; United States Geological Survey: Reston, VA, USA, 1997; p. 93.
- Harrington, J.M.; Fendorf, S.E.; Rosenzweig, R.F. Biotic generation of arsenic(III) in metal(loid)-contaminated freshwater lake sediments. Environ. Sci. Technol. 1998, 32, 2425–2430. [Google Scholar] [CrossRef]
- Harrington, J.M.; LaForce, M.J.; Rember, W.C.; Fendorf, S.E.; Rosenzweig, R.F. Phase associations and mobilization of iron and trace elements in Coeur d’Alene Lake, Idaho. Environ. Sci. Technol. 1998, 32, 650–656. [Google Scholar] [CrossRef]
- Pedersen, T.F. A comment on the future environmental status of Coeur d’Alene Lake, Idaho. Northwest Sci. 1996, 70, 179–182. [Google Scholar]
- Şengör, S.S.; Spycher, N.F.; Ginn, T.R.; Sani, R.K.; Peyton, B. Biogeochemical reactive–diffusive transport of heavy metals in Lake Coeur d’Alene sediments. Appl. Geochem. 2007, 22, 2569–2594. [Google Scholar] [CrossRef]
- Child, A.W.; Moore, B.C.; Vervoort, J.D.; Beutel, M.W. Bioavailability and uptake of smelter emissions in freshwater zooplankton in northeastern Washington, USA lakes using Pb isotope analysis and trace metal concentrations. Environ. Pollut. 2018, 238, 348–358. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Box, S.E.; Tonkin, J.W. Modeling precipitation and sorption of elements during mixing of river water and porewater in the Coeur d’Alene River Basin. Environ. Sci. Technol. 2003, 37, 4694–4701. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Blank, R.G. Dissolved and labile concentrations of Cd, Cu, Pb, and Zn in the South Fork Coeur d’Alene River, Idaho: Comparisons among chemical equilibrium models and implications for biotic ligand models. Appl. Geochem. 2008, 23, 3355–3371. [Google Scholar] [CrossRef]
- Dockrey, J.W.; Lindsay, M.B.J.; Mayer, K.U.; Beckie, R.D.; Norlund, K.L.I.; Warren, L.A.; Southam, G. Acidic microenvironments in waste rock characterized by neutral drainage: Bacteria–mineral interactions at sulfide surfaces. Minerals 2014, 4, 170–190. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Kan, A.T.; Tomson, M.B. Critical evaluation of desorption phenomena of heavy metals from natural sediments. Environ. Sci. Technol. 2003, 37, 5566–5573. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W. Chemistry of iron sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef] [PubMed]
- Schaider, L.A.; Senn, D.B.; Estes, E.R.; Brabander, D.J.; Shine, J.P. Sources and fates of heavy metals in a mining-impacted stream: Temporal variability and the role of iron oxides. Sci. Total Environ. 2014, 490, 456–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schippers, A. Biogeochemistry of metal sulfide oxidation in mining environments, sediments, and soils. Geol. Soc. Am. Spec. Pap. 2004, 379, 49–62. [Google Scholar] [CrossRef]
- Toevs, G.; Morra, M.J.; Winowiecki, L.; Strawn, D.; Polizzotto, M.L.; Fendorf, S. Depositional influences on porewater arsenic in sediments of a mining-contaminated freshwater lake. Environ. Sci. Technol. 2008, 42, 6823–6829. [Google Scholar] [CrossRef]
- Toevs, G.R.; Morra, M.J.; Polizzotto, M.L.; Strawn, D.G.; Bostick, B.C.; Fendorf, S. Metal(loid) diagenesis in mine-impacted sediments of Lake Coeur d’Alene, Idaho. Environ. Sci. Technol. 2006, 40, 2537–2543. [Google Scholar] [CrossRef]
- Bostick, B.C.; Hansel, C.M.; Fendorf, S. Seasonal fluctuations in zinc speciation within a contaminated wetland. Environ. Sci. Technol. 2001, 35, 3823–3829. [Google Scholar] [CrossRef]
- Moberly, J.; D’Imperio, S.; Parker, A.; Peyton, B. Microbial community signature in Lake Coeur d’Alene: Association of environmental variables and toxic heavy metal phases. Appl. Geochem. 2016, 66, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Moberly, J.G.; Borch, T.; Sani, R.K.; Spycher, N.F.; Şengör, S.S.; Ginn, T.R.; Peyton, B.M. Heavy metal–mineral associations in Coeur d’Alene River sediments: A synchrotron-based analysis. Water. Air. Soil Pollut. 2008, 201, 195–208. [Google Scholar] [CrossRef]
- Boujelben, N.; Bouzid, J.; Elouear, Z. Adsorption of nickel and copper onto natural iron oxide-coated sand from aqueous solutions: Study in single and binary systems. J. Hazard. Mater. 2009, 163, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M. Simultaneous incorporation of Mn, Ni and Co in the goethite (alpha -FeOOH) structure. Clay Miner. 1991, 26, 427–430. [Google Scholar] [CrossRef]
- Galán, E.; Gómez-Ariza, J.L.; González, I.; Fernández-Caliani, J.C.; Morales, E.; Giráldez, I. Heavy metal partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt. Appl. Geochem. 2003, 18, 409–421. [Google Scholar] [CrossRef]
- Chen, K.L.; Elimelech, M. Influence of humic acid on the aggregation kinetics of fullerene (C60) nanoparticles in monovalent and divalent electrolyte solutions. J. Colloid Interface Sci. 2007, 309, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Filella, M.; Buffle, J. Factors controlling the stability of submicron colloids in natural waters. In Colloids in the Aquatic Environment; Tadros, T.F., Gregory, J., Eds.; Elsevier: Oxford, UK, 1993; pp. 255–273. ISBN 978-1-85861-038-2. [Google Scholar]
- Hyung, H.; Fortner, J.D.; Hughes, J.B.; Kim, J.-H. Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ. Sci. Technol. 2007, 41, 179–184. [Google Scholar] [CrossRef]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef]
- Pizarro, J.; Belzile, N.; Filella, M.; Leppard, G.G.; Negre, J.-C.; Perret, D.; Buffle, J. Coagulation/sedimentation of submicron iron particles in a eutrophic lake. Water Res. 1995, 29, 617–632. [Google Scholar] [CrossRef]
- National Research Council. Superfund and Mining Megasites: Lessons from the Coeur d’Alene River Basin; The National Academies of Sciences, Engineering, Medicine: Washington, DC, USA, 2005; p. 484.
- Long, K.R. Production and Disposal of Mill Tailings in the Coeur d‘Alene Mining Region, Shoshone County, Idaho; Preliminary Estimates; United States Geological Survey: Reston, VA, USA, 1998.
- Balistrieri, L.S.; Box, S.E.; Bookstrom, A.A.; Hooper, R.L.; Mahoney, J.B. Impacts of Historical Mining in the Coeur d‘Alene River Basin; Bulletin; United States Geological Survey: Reston, VA, USA, 2010.
- Clark, G.M.; Mebane, C.A. Sources, Transport, and Trends for Selected Trace Metals and Nutrients in the Coeur d’Alene and Spokane River Basins, Northern Idaho, 1990–2013; United States Geological Survey: Reston, VA, USA, 2014; p. 62.
- Tonkin, J.W.; Balistrieri, L.S.; Murray, J.W. Modeling metal removal onto natural particles formed during mixing of acid rock drainage with ambient surface water. Environ. Sci. Technol. 2002, 36, 484–492. [Google Scholar] [CrossRef]
- Holliday, V.T. Methods of soil analysis, part 1, physical and mineralogical methods. Geoarchaeology 1990, 5, 87–89. [Google Scholar] [CrossRef]
- Gavlak, R.; Horneck, D.; Miller, R.O. Soil, Plant, and Water Reference Methods for the Western Region. In Proceedings of the WERA-103 Technical Committee, 3rd ed.; Oregon State University: Corvallis, OR, USA, 2005; Volume WREP 125. [Google Scholar]
- Sims, J.R.; Haby, V.A. Simplified colorimetric determination of soil organic matter. Soil Sci. 1971, 101, 137–141. [Google Scholar] [CrossRef]
- Schippers, A.; Sand, W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl. Environ. Microbiol. 1999, 65, 319–321. [Google Scholar] [CrossRef] [Green Version]
- Druschel, G.K.; Hamers, R.J.; Banfield, J.F. Kinetics and mechanism of polythionate oxidation to sulfate at low pH by O2 and Fe3+. Geochim. Cosmochim. Acta 2003, 67, 4457–4469. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Chen, Y.; Zhao, C.; Zhang, Y.; Ke, B. Interactions of oxygen and water molecules with pyrite surface: A new insight. Langmuir 2018, 34, 1941–1952. [Google Scholar] [CrossRef]
- Terranova, U.; Mitchell, C.; Sankar, M.; Morgan, D.; de Leeuw, N.H. Initial oxygen incorporation in the prismatic surfaces of troilite FeS. J. Phys. Chem. C 2018, 122, 12810–12818. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Hu, Y.; Wang, Z.; Chen, J.; Niu, X.; Li, Y.; Gong, X. HO selective cleavage FeS bond for FeS2 electrolysis in alkaline solution. Electrochimica Acta 2019, 306, 327–338. [Google Scholar] [CrossRef]
- Findlay, A.J.; Kamyshny, A. Turnover rates of intermediate sulfur species (Sx2-, S0, S2O32−, S4O62−, SO32−) in anoxic freshwater and sediments. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Langman, J.B.; Blowes, D.W.; Sinclair, S.A.; Krentz, A.; Amos, R.T.; Smith, L.J.D.; Pham, H.N.; Sego, D.C.; Smith, L. Early evolution of weathering and sulfide depletion of a low-sulfur, granitic, waste rock in an Arctic climate: A laboratory and field site comparison. J. Geochem. Explor. 2015, 156, 61–71. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Heap, M.J.; Reuschlé, T.; Farquharson, J.I.; Baud, P. Permeability of volcanic rocks to gas and water. J. Volcanol. Geotherm. Res. 2018, 354, 29–38. [Google Scholar] [CrossRef]
- Jensen, L.C.; Becerra, J.R.; Escudey, M. Impact of physical/chemical properties of volcanic ash-derived soils on mechanisms involved during sorption of ionisable and non-ionisable herbicides. Adv. Sorpt. Process Appl. 2018. [Google Scholar] [CrossRef] [Green Version]
- Huettel, M.; Røy, H.; Precht, E.; Ehrenhauss, S. Hydrodynamical impact on biogeochemical processes in aquatic sediments. In The Interactions between Sediments and Water; Kronvang, B., Ed.; Springer: Dordrecht, The Netherlands, 2003; pp. 231–236. [Google Scholar]
- Bryant, W.R. Permeability of clays, silty-clays and clayey-silts. Gulf Coast Assoc. Geol. Soc. Trans. 2002, 52, 1069–1077. [Google Scholar]
- Behyan, S.; Hu, Y.; Urquhart, S.G. Sulfur 1s near-edge X-ray absorption fine structure (NEXAFS) of thiol and thioether compounds. J. Chem. Phys. 2011, 134, 244304. [Google Scholar] [CrossRef] [PubMed]
- Sham, T.K. Nanoparticles and nanowires: Synchrotron spectroscopy studies. Int. J. Nanotechnol. 2008, 5, 1194–1246. [Google Scholar] [CrossRef]
- Alp, E.E.; Mini, S.M.; Ramanathan, M. X-ray Absorption Spectroscopy: EXAFS and XANES-A Versatile Tool to Study the Atomic and Electronic Structure of Materials; Argonne National Laboratory: Lemont, IL, USA, 1990; pp. 25–36.
- Penner-Hahn, J.E. X-ray absorption spectroscopy. eLS 2005. [Google Scholar] [CrossRef]
- Yang, Y.; Harmer, S.; Chen, M. Synchrotron-based XPS and NEXAFS study of surface chemical species during electrochemical oxidation of chalcopyrite. Hydrometallurgy 2015, 156, 89–98. [Google Scholar] [CrossRef]
- Koningsberger, D.C.; Ramaker, D.E. Applications of X-ray Absorption Spectroscopy in Heterogeneous Catalysis: EXAFS, Atomic XAFS, and Delta XANES. In Handbook of Heterogeneous Catalysis; American Cancer Society: Atlanta, GA, USA, 2008; pp. 774–803. ISBN 978-3-527-61004-4. [Google Scholar]
- Koningsberger, D.C.; Mojet, B.L.; van Dorssen, G.E.; Ramaker, D.E. XAFS spectroscopy; fundamental principles and data analysis. Top. Catal. 2000, 10, 143–155. [Google Scholar] [CrossRef]
- Newville, M. Fundamentals of XAFS. Rev. Mineral. Geochem. 2014, 78, 33–74. [Google Scholar] [CrossRef]
- Steudel, R. Properties of sulfur-sulfur bonds. Angew. Chem. Int. 1975, 14, 655–664. [Google Scholar] [CrossRef]
- Dos Santos, E.C.; Lourenço, M.P.; Pettersson, L.G.M.; Duarte, H.A. Stability, structure, and electronic properties of the pyrite/arsenopyrite solid–solid interface—A DFT study. J. Phys. Chem. C 2017, 121, 8042–8051. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, E.C.; de Mendonça Silva, J.C.; Duarte, H.A. Pyrite oxidation mechanism by oxygen in aqueous medium. J. Phys. Chem. C 2016, 120, 2760–2768. [Google Scholar] [CrossRef] [Green Version]
- El Jaroudi, O.; Picquenard, E.; Demortier, A.; Lelieur, J.-P.; Corset, J. Polysulfide anions. 1. Structure and vibrational spectra of the S22- and S32- anions. Influence of the cations on bond length and angle. Inorg. Chem. 1999, 38, 2394–2401. [Google Scholar] [CrossRef]
- Nikoo, S.; Meister, P.J.; Hayward, J.J.; Gauld, J.W. An assessment of computational methods for calculating accurate structures and energies of bio-relevant polysulfur/selenium-containing compounds. Molecules 2018, 23, 3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawthorne, F.C.; Krivovichev, S.V.; Burns, P.C. The crystal chemistry of sulfate minerals. Rev. Mineral. Geochem. 2000, 40, 1–112. [Google Scholar] [CrossRef]
- Bradley, A.S.; Leavitt, W.D.; Johnston, D.T. Revisiting the dissimilatory sulfate reduction pathway. Geobiology 2011, 9, 446–457. [Google Scholar] [CrossRef]
- Plugge, C.M.; Zhang, W.; Scholten, J.C.M.; Stams, A.J.M. Metabolic flexibility of sulfate-reducing bacteria. Front. Microbiol. 2011, 2. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.A.; Venceslau, S.S.; Grein, F.; Leavitt, W.D.; Dahl, C.; Johnston, D.T.; Pereira, I.A.C. A protein trisulfide couples dissimilatory sulfate reduction to energy conservation. Science 2015, 350, 1541–1545. [Google Scholar] [CrossRef]
- Canfield, D.E. Sulfate reduction and oxic respiration in marine sediments: Implications for organic carbon preservation in euxinic environments. Deep Sea Res. Part Oceanogr. Res. Pap. 1989, 36, 121–138. [Google Scholar] [CrossRef] [Green Version]
- Holmer, M.; Storkholm, P. Sulphate reduction and sulphur cycling in lake sediments: A review. Freshw. Biol. 2001, 46, 431–451. [Google Scholar] [CrossRef]
- Lovley, D.R. Fe(III) and Mn(IV) reduction. Environ. Microbe-Met. Interact. 2000, 3–30. [Google Scholar] [CrossRef]
- Lovley, D.R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Mol. Biol. Rev. 1991, 55, 259–287. [Google Scholar] [CrossRef] [Green Version]
- Nealson, K.H.; Myers, C.R. Microbial reduction of manganese and iron: New approaches to carbon cycling. Appl. Environ. Microbiol. 1992, 58, 439–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bretschger, O.; Obraztsova, A.; Sturm, C.A.; Chang, I.S.; Gorby, Y.A.; Reed, S.B.; Culley, D.E.; Reardon, C.L.; Barua, S.; Romine, M.F.; et al. Current production and metal oxide reduction by shewanella oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 2007, 73, 7003–7012. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R.; Giovannoni, S.J.; White, D.C.; Champine, J.E.; Phillips, E.J.P.; Gorby, Y.A.; Goodwin, S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 1993, 159, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Hem, J.D. Chemical factors that influence the availability of iron and manganese in aqueous systems. GSA Bull. 1972, 83, 443–450. [Google Scholar] [CrossRef]









| Depth (cm) | Sand | Silt | Clay | Phosphorus | Nitrate (as N) | Ammonia (as N) | Organic Matter |
|---|---|---|---|---|---|---|---|
| Units | % 1 | % | % | µg/g 2 | µg/g | µg/g | % |
| 0 to 5 | 12.5 | 65.6 | 21.9 | 24 | 0.80 | 130 | 5.2 |
| 5 to 10 | 13.6 | 57.6 | 28.8 | 23 | <0.72 | 77 | 3.3 |
| 10 to 15 | 7.0 | 60.8 | 32.3 | 21 | <0.72 | 56 | 2.8 |
| 15 to 20 | 6.1 | 59.2 | 34.7 | 20 | <0.72 | 69 | 3.7 |
| 20 to 25 | 11.5 | 59.0 | 29.5 | 16 | 0.79 | 84 | 4.2 |
| 25 to 30 | 7.9 | 66.1 | 26.0 | 15 | <0.72 | 130 | 4.8 |
| 30 to 35 | 17.3 | 56.7 | 26.0 | 25 | <0.72 | 120 | 4.9 |
| 35 to 40 | 11.4 | 60.4 | 28.2 | 25 | <0.72 | 120 | 4.2 |
| 40 to 45 | 14.7 | 59.1 | 26.2 | 27 | 0.79 | 120 | 3.8 |
| 45 to 50 | 16.0 | 52.5 | 31.5 | 24 | <0.72 | 130 | 4.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langman, J.B.; Ali, J.D.; Child, A.W.; Wilhelm, F.M.; Moberly, J.G. Sulfur Species, Bonding Environment, and Metal Mobilization in Mining-Impacted Lake Sediments: Column Experiments Replicating Seasonal Anoxia and Deposition of Algal Detritus. Minerals 2020, 10, 849. https://doi.org/10.3390/min10100849
Langman JB, Ali JD, Child AW, Wilhelm FM, Moberly JG. Sulfur Species, Bonding Environment, and Metal Mobilization in Mining-Impacted Lake Sediments: Column Experiments Replicating Seasonal Anoxia and Deposition of Algal Detritus. Minerals. 2020; 10(10):849. https://doi.org/10.3390/min10100849
Chicago/Turabian StyleLangman, Jeff B., Jaabir Duunya Ali, Andrew W. Child, Frank M. Wilhelm, and James G. Moberly. 2020. "Sulfur Species, Bonding Environment, and Metal Mobilization in Mining-Impacted Lake Sediments: Column Experiments Replicating Seasonal Anoxia and Deposition of Algal Detritus" Minerals 10, no. 10: 849. https://doi.org/10.3390/min10100849