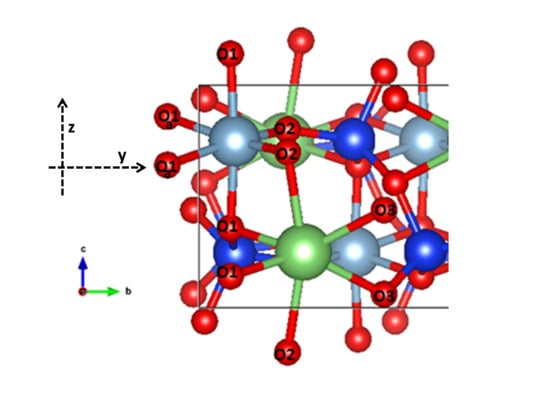
Some Complementary Data about the Spectroscopic Properties of Manganese Ions in Spodumene Crystals
Abstract
:1. Introduction
- (1)
- For Mn2+: what is the lattice site of Mn2+ and what are the crystal-field parameters of this ion?
- (2)
- For Mn3+: the problem of color change during irradiation and heating?
- (a)
- Whether Mn3+ in spodumene crystal changes into Mn4+ after irradiation;
- (b)
- How the crystal-field splitting parameter (Dq) and crystal-field stabilization energy (CFSE) of Mn3+ change after irradiation and what the symmetry of Mn3+ site is (tetragonal or monoclinic);
- (c)
- Whether Mn3+ exists in the M1 or the M2 site; which one is the case for natural crystals, and which one is for artificial pink spodumene crystals?
2. Materials and Methods
3. Results and Discussion
3.1. Chemistry of Studied Spodumene Crystals
3.2. Irradiation and Heating Effect
3.3. Spectroscopy of Mn2+ in Spodumene
3.4. Spectroscopy of Mn3+ in Spodumene
- (a)
- First question: does Mn3+ in the studied spodumene samples change its valence due to irradiation?
- (b)
- Second issue: what is the effective symmetry of the crystal field—tetragonal, as was proposed by Ito et al. [21], or monoclinic?
- υ1: 5B1g(5E) →5A1g(5E) – 1630 nm, i.e., 6135 cm−1 = 4 · Ds + 5 · Dt
- υ2: 5B1g(5E) →5B2g(5T2) – 624 nm, i.e., 16,025 cm−1 = 10 · Dq
- υ3: 5B1g(5E) →5Eg(5T2) – 529 nm, i.e., 18,904 cm−1 = 3 · Ds − 5 · Dt + 10 · Dq
- υ1: 5B1(5E) → 5A1(5Eg) were not observed and a value of 6000 cm−1 can be assumed for it;
- υ2: 5B1(5E) → 5A1(5T2g) at 624 nm (16,025 cm−1) and 628 nm (15,923 cm−1);
- υ3: 5B1(5E) → 5B2(5T2g) at 529 nm (18,904 cm−1) and 536 nm (18,657 cm−1);
- υ4: 5B1(5E) → 5A2(5T2g) at 440 nm (22,727 cm−1) and 447 nm (22,371 cm−1).
- (c)
- Does Mn3+ exist in M1 or M2 site?
4. Conclusions
- After X-ray irradiation, over 24 h, the spodumene crystals, whether colorless or pink, changed their color to green, but without changing the Mn3+ valence to Mn4+. Therefore, the presence of Mn4+ in the irradiated sample was excluded.
- It is very likely that Mn3+ occupies the M2 site of monoclinic symmetry. According to the absorption transitions, the changes in 10Dq and in CFSE values for the irradiated crystal are not very significant, and the color change process can be reversed.
- Mn3+ can occupy the M1 site if is introduced into a spodumene lattice during crystal growth. The absorption spectrum of such a crystal exhibited one distinct band at 530–540 nm, and the emission of the Mn2+ present in this site is measured at 625 nm.
- For other pink spodumene crystals, the absorption spectra as at Figure 4a,b are appropriate for manganese ions (2+) and (3+) present in the M2 site, and its coloration was caused artificially. Such crystals initially contained only Mn2+. Some of these ions changed their valence to (3+) due to irradiation and heating.
- Spodumene is a crystal that easily changes color from pink to green and can easily create optically active point defects in it. For this reason, it can be used in optics as a material with the possibility of modulating emission colors.
Author Contributions
Funding
Conflicts of Interest
References
- Tribaudino, M.; Nestola, F.; Prencipe, M.; Rundolf, H. A single-crystal neutron-diffraction investigation of spodumene at 54 K. Can. Mineral. 2003, 41, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.; Gibbs, G.; Ribbe, P.H. Quadratic elongation: A quantitative measure of distortion in coordination polyhedra. Science 1971, 172, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D.; Shannon, R.D. Empirical bond-strength-bond-length curves for oxides. Acta Crystall. 1973, A29, 266–282. [Google Scholar] [CrossRef]
- Bakhin, A.I.; Manapov, R.A. Investigation of clinopyroxenes by optical and Mössbauer spectroscopy. Geochem. Int. 1976, 13, 81–88. (In Russian) [Google Scholar]
- Platonov, A.N.; Taran, M.N.; Balitsky, V.S. Nature of Colour of Gems; Niedra: Moscow, Russia, 1984. (In Russian) [Google Scholar]
- Fuju, A.T.; Isotani, S. Optical absorption study of five varieties of spodumene. An. Acad. Bras. Ciênc. 1988, 60, 127–135. [Google Scholar]
- De Grave, E.; Van Alboom, A.; Eeckhout, S.G. Electronic and magnetic properties of a natural aegirine as observed from its Mössbauer spectra. Phys. Chem. Miner. 1998, 25, 378–388. [Google Scholar] [CrossRef]
- Schmidbauer, E.; Kunzmann, T. Electrical conductivity, thermopower and 57Fe Mössbauer spectroscopy of aegirine (NaFeSi2O6). Phys. Chem. Miner. 2004, 31, 102–114. [Google Scholar] [CrossRef]
- Filip, J.; Novák, M.; Beran, A.; Zbořil, R. Crystal chemistry and OH defect concentrations in spodumene from different granitic pegmatites. Phys. Chem. Miner. 2006, 32, 733–746. [Google Scholar] [CrossRef]
- Katerinopoulou, A.; Grodzicki, M.; Tippelt, G. Mössbauer spectroscopy and molecular orbital calculations on iron bearing omphacite. Miner. Petrol. 2013, 107, 281–287. [Google Scholar] [CrossRef]
- Czaja, M.; Lisiecki, R.; Kądziołka-Gaweł, M.; Winiarski, A.; Krzykawski, T. The afterglow effect of Mn-bearing natural LiAlSi2O6 spodumene crystals. Opt. Mater. 2019, 96, 109321. [Google Scholar] [CrossRef]
- Burns, R. Mineralogical Applications of Crystal Field Theory; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Khomenko, V.M.; Platonov, A.N. Electronic absorption spectra of Cr3+ ions in natural clinopyroxenes. Phys. Chem. Miner. 1985, 11, 261–265. [Google Scholar] [CrossRef]
- Walker, G.; El Jaer, A.; Sherlock, R.; Glynn, T.J.; Czaja, M.; Mazurak, Z. Luminescence spectroscopy of Cr3+ and Mn2+ in spodumene (LiAlSi2O6). J. Lumin. 1997, 72–74, 27–280. [Google Scholar] [CrossRef]
- O’Bannon, E.; Williams, Q. A Cr3+ luminescence study of spodumene: Effects of site geometry, phase transition, and a level-crossing. Am. Mineral. 2016, 101, 1406–1413. [Google Scholar] [CrossRef]
- Chadwick, K.M.; Shen, A.H.; Laurs, B.M.; Simmons, W.B.; Falser, A.U. Cr/V-bearing green spodumene from Afghanistan. Gems. Gemol. 2007, 43, 265. [Google Scholar]
- Chandrasekhar, B.K.; White, W.B. Polarized luminescence spectra of kunzite. Phys. Chem. Miner. 1992, 18, 433–440. [Google Scholar] [CrossRef]
- Claffy, E.W. Composition, tenebrescence and luminescence of spodumene minerals. Am. Mineral. 1953, 38, 919–931. [Google Scholar]
- Platonov, A.N. Priroda Okraski Minieralow; Naukova Dumka: Kiev, Ukraine, 1976. (In Russian) [Google Scholar]
- Nassau, K. The Physics and Chemistry of Color—The Fifteen Causes of Color; John Wiley and Sons: NewYork, NY, USA, 1983; pp. 201–202. [Google Scholar]
- Ito, A.S.; Isotani, S. Heating effects on the optical absorption spectra of irradiated, natural spodumene. Radiat. Effects Defects Solids 1991, 116, 307–314. [Google Scholar] [CrossRef]
- Souza, S.O.; Watanabe, S.; Lima, A.F.; Lalic, M.V. Thermoluminescence mechanism in lilac luminescence. Acta Phys. Pol. Ser. A 2007, 112, 1001–1006. [Google Scholar] [CrossRef]
- Schmitz, B.; Lehmann, G. Color Centers of Manganese in Natural Spodumene LiAlSi2O6. Ber. Bunsenges. Phys. Chem. 1975, 79, 1044–1049. [Google Scholar] [CrossRef]
- Oliveira, R.A.P.; Mello, A.C.S.; Lima, H.R.B.R.; Santos Campos, S.; Souza, S.O. Radiation detection using the color changes of lilac spodumene. In Proceedings of the International Nuclear Atlantic Conference—INAC 2009, Rio de Janeiro, RJ, Brazil, 27 September–2 October 2009; ISBN 978-85-99141-03-8. [Google Scholar]
- Curie, D.; Barthou, C.; Canny, B. Covalent bonding of Mn2+ ions in octahedral and tetrahedral coordination. J. Chem. Phys. 1974, 61, 3048–3062. [Google Scholar] [CrossRef]
- Fridrichová, J.; Bačík, P.; Ertl, A.; Wildner, M.; Dekan, J.; Miglierini, M. Jahn-Teller distortion of Mn3+-occupied octahedra in red beryl from Utah indicated by optical spectroscopy. J. Mol. Struct. 2018, 1152, 79–86. [Google Scholar] [CrossRef]
- Czaja, M.; Lisiecki, R.; Chrobak, A.; Sitko, R.; Mazurak, Z. The absorption-and luminescence spectra of Mn3+ in beryl and vesuvianite. Phys. Chem. Miner. 2018, 45, 475–488. [Google Scholar] [CrossRef] [Green Version]
- Isotani, S.; Watari, K.; Mizukami, A.; Bonventi, W., Jr.; Ito, A.S. UV optical absorption analysis of spodumene crystals from Brazil. Physica B 2007, 391, 322–330. [Google Scholar] [CrossRef]
- Manning, P.G. Absorption spectra of the manganese bearing chain silicates pyroxmangite, rhodonite, bustamite and serandite. Can. Mineral. 1968, 9, 348–357. [Google Scholar]
- Manning, P.G. Racah parameters and their relationship to lengths and covalencies of Mn2+- and Fe3+-oxygen bonds in silicates. Can. Mineral. 1970, 10, 677–688. [Google Scholar]
- Smith, G.; Halenius, U.; Langer, K. Low temperature spectral studies of Mn3+- bearing andalusite and epidote type minerals in the range 30,000–5000 cm. Phys. Chem. Miner. 1982, 8, 136–142. [Google Scholar] [CrossRef]
- Stedman, G.E. Interpretation of the Crystal Field for V2+, Cr3+, and Mn4+ in Corundum. J. Chem. Phys. 1969, 51, 4123–4125. [Google Scholar] [CrossRef]
- Suchocki, A.; Allen, J.D.; Powell, R.C. Spectroscopy and four-wave mixing in Li4Ge5O12:Mn4+ crystals. Phys. Rev. B 1987, 36, 6729–6734. [Google Scholar] [CrossRef]
- Brik, M.G.; Sildos, I.; Berkowski, M.; Suchocki, A. Spectroscopic and crystal field studies of YAlO3 single crystals doped with Mn ions. J. Phys. Condens. Matter. 2009. [Google Scholar] [CrossRef]
- Zhydachevskii, Y.; Galanciak, D.; Kobyakov, S.; Berkowski, M.; Kamińska, A.; Suchocki, A.; Zakharko, Y.; Durygin, A. Photoluminescence studies of Mn4+ ions in YAlO3 crystals at ambient and high pressure. J. Phys. Condens. Matter. 2006, 18, 11385–11396. [Google Scholar] [CrossRef]
- Gaft, M.; Stręk, W.; Nagli, L.; Panczer, G.; Rossman, G.R.; Marciniak, L. Laser-induced time-resolved luminescence of natural sillimanite Al2SiO5 and syntheticAl2SiO5 activated by chromium. J. Lumin. 2012, 132, 2855–2862. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, F.; Cao, C.; Yu, X.; Liang, A.; Guo, S.; Xue, H. Synthesis and luminescence properties of CaAl2O4:Mn4+ phosphor. Opt. Mater. 2014, 38, 53–56. [Google Scholar] [CrossRef]
- Erdem, U.; Esra, Ö.; Kalaycioglu Ozpozan, N.; Karacaoglu, E. Thermoluminescence and photoluminescence properties of Mn4+, Pr3+,4+, Nd4+ and Eu3+ in MgAl2Si2O8. J. Lumin. 2016, 173, 73–81. [Google Scholar] [CrossRef]
- Yu, K.M.; Walukiewicz, W.; Muto, S.; Jin, H.C. The effects of x-ray induced structural changes on the microstructure of a-Si after thermal crystallization. Appl. Phys. Lett. 1999, 75, 2032–2034. [Google Scholar] [CrossRef]
- He, G.C.; Xiang, H.M.; Jiang, W.; Kang, Q.; Chen, J.H. First-principles theory on electronic structure and floatability of spodumene. Rare. Met. 2014, 33, 742–748. [Google Scholar] [CrossRef]
- Sherman, D.M.; Vergo, N. Optical spectrum, site occupancy, and oxidation state of Mn in montmorillonite. Am. Mineral. 1988, 73, 140–144. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Brik, M.G.; Teng, H.; Lin, H.; Zhou, S.; Avram, N.M. Spectroscopic and crystal field studies of LiAlO2:Mn2+ single crystals. J. Alloys Compd. 2010, 506, 4–9. [Google Scholar] [CrossRef]







| Oxide Content | Sample 3 | Sample 14 | Sample 13 | Sample 36a |
|---|---|---|---|---|
| Major chemical constituents (wt%) | ||||
| SiO2 a | 64.510 | 64.48 | 64.46 | 64.44 |
| Al2O3 a | 27.280 | 27.25 | 27.19 | 27.28 |
| Li2O * | 8.016 | 7.970 | 7.885 | 8.028 |
| Na2O3 b | 0.110 | 0.13 | 0.11 | 0.090 |
| FeO b | 0.025 | 0.09 | 0.27 | 0.130 |
| MnO b | 0.059 | 0.079 | 0.085 | 0.032 |
| Trace element contents from ICP-MS (ppm) | ||||
| Element (MDL **) | ||||
| Mn (2) | 459 | 612 | 663 | 250 |
| Fe (20) | 200 | 700 | 2100 | 200 |
| Be (1) | 16 | 17 | 16 | 15 |
| Sc (0.1) | 0.9 | 1.1 | 1.1 | 1.1 |
| Na (20) | 820 | 990 | 840 | 660 |
| Sc (0.1) | 0.9 | 1.1 | 1.1 | 1.1 |
| Ti (10) | 20 | 30 | 20 | 20 |
| Cr (1) | 3 | 4 | 4 | 4 |
| Cu (0.02) | 2.27 | 0.74 | 0.61 | 1.52 |
| Zn (0.2) | 1.3 | 17.5 | 11.4 | 26.3 |
| Ga (0.1) | 67.87 | 64.80 | 64.93 | 46.18 |
| Rb (0.1) | 3.3 | 4.4 | 0.2 | 0.5 |
| Cd (0.02) | 0.07 | 0.06 | 0.14 | 0.10 |
| Sn (0.1) | 109.7 | 157.2 | 132.5 | 129.0 |
| Cs (0.1) | 4.3 | 1.0 | 0.3 | 0.7 |
| W (0.1) | >200 | 15.5 | 0.7 | 11.1 |
| Pb (0.02) | 0.93 | 0.26 | 0.49 | 1.39 |
| sum (from Be to Pb) | 1251 | 1305 | 1094 | 918 |
| Bands | Transitions | Energy of Transitions | |||
|---|---|---|---|---|---|
| Measured at T = 300 K | Calculated (cm−1) | ||||
| (nm) | (cm−1) | Tanabe-Sugano a | Curie et al. [25] b | ||
| υ1 | 6A1(S) → 4T1g(4G) | 483 | 20,700 | 15,686 | 19,040 |
| υ2 | 6A1(S) → 4T2g(4G) | 431 | 23,200 | 19,139 | 20,855 |
| υ3 | 6A1(S) → 4Eg(4G) | 408 | 24,510 | 24,038 | 23,400 |
| υ3 | 6A1(S) → 4A1g(4G) | 415 | 24,096 | 23,185 | |
| Δ(4A, 4E) | 414 | 215 | |||
| υ4 | 6A1(S) → 4T2g(D) | 360 | 27,777 | ? | 28,650 |
| υ5 | 6A1(S) → 4Eg(D) | 333 | 30,030 | 30,300 | 32,800 |
| Spectroscopic Parameters | Spodumene Crystals | ||||
|---|---|---|---|---|---|
| Units | Current Study | [21] | |||
| (3) Pink Primary | (3) Green X-ray Irradiated | Lilac Primary | Green γ−Irradiated | ||
| 5B1g(5E) →5A1g(5E) | (nm) | 1630 | 1630 | 870 | 952 |
| (cm−1) | 6135 | 6135 | 11,500 | 10,500 | |
| 5B1g(5E) →5B2g(5T2) | (nm) | 624 | 628 | 537 | 641 |
| (cm−1) | 16,025 | 15,923 | 18,600 | 15,600 | |
| 5B1g(5E) →5Eg(5T2) | (nm) | 529 | 536 | 454 | 465 |
| (cm−1) | 18,904 | 18,656 | 22,000 | 21.500 | |
| Dq | (cm−1) | 1603 | 1592 | 1860 | 1560 |
| CSFE | 11,083 | 11,025 | 16,910 | 14,610 | |
| Dt | 197 | 213 | 597 | 226 | |
| Ds | 1288 | 1267 | 2129 | 2343 | |
| Dq(z) | 1833 | 1592 | 1164 | 1296 | |
| Dq(xy) | 1488 | 1219 | 2208 | 1692 | |
| - | 1.04 | 1.05 | 0.879 | 0.948 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaja, M.; Lisiecki, R.; Kądziołka-Gaweł, M.; Winiarski, A. Some Complementary Data about the Spectroscopic Properties of Manganese Ions in Spodumene Crystals. Minerals 2020, 10, 554. https://doi.org/10.3390/min10060554
Czaja M, Lisiecki R, Kądziołka-Gaweł M, Winiarski A. Some Complementary Data about the Spectroscopic Properties of Manganese Ions in Spodumene Crystals. Minerals. 2020; 10(6):554. https://doi.org/10.3390/min10060554
Chicago/Turabian StyleCzaja, Maria, Radosław Lisiecki, Mariola Kądziołka-Gaweł, and Antoni Winiarski. 2020. "Some Complementary Data about the Spectroscopic Properties of Manganese Ions in Spodumene Crystals" Minerals 10, no. 6: 554. https://doi.org/10.3390/min10060554