The Impact of Ecological Restoration on Biogeochemical Cycling and Mercury Mobilization in Anoxic Conditions on Former Mining Sites in French Guiana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Initial Soil Sample Characterization
2.2.1. Soil Total Major Element Content
2.2.2. Soil Total Mercury Content
2.2.3. Soil Carbon, Nitrogen and Phosphorus Measurements
2.2.4. Total Organic Carbon in Soil Samples
2.3. Experimental Conditions
2.3.1. Bacterial Metabolism
2.3.2. Iron Reduction in Culture Medium
2.3.3. Total Iron, Aluminum and Manganese in the Culture Medium
2.4. Statistical Analysis
2.5. Quality Assurance and Control (QA/QC)
3. Results
3.1. Soil Physical and Chemical Properties
3.2. Sulfate and Sulfide Concentration in the Culture Medium
3.3. Heterotrophic Metabolism and Dissolved Carbon in the Culture Medium
3.4. Metals Solubilization
3.4.1. Iron in the Culture Medium
3.4.2. Aluminum in the Culture Medium
3.4.3. Manganese in the Culture Medium
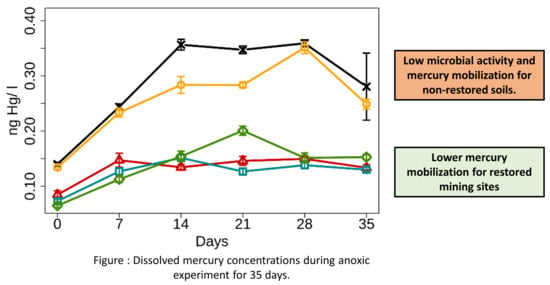
3.4.4. Mercury in the Culture Medium
3.4.5. pH in the Culture Medium
3.5. Principal Component Analysis of Microbial Reducing Activities and Metal Solubilization
4. Discussion
4.1. Properties of the Rehabilitated Soils and Distribution of the Various Trace Elements
4.1.1. Soil Texture of Rehabilitated Soils
4.1.2. Mercury Content of Rehabilitated Soils
4.2. Microbial Activities during the Anoxic Soil Experiment
4.2.1. Heterotrophic Activities and Iron Reduction
4.2.2. Sulfate-Reducing Activities
4.3. Mercury Dissolution during the Anoxic Soil Experiment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veiga, M.M.; Maxson, P.A.; Hylander, L.D. Origin and consumption of mercury in small-scale gold mining. J. Clean. Prod. 2006, 14, 436–447. [Google Scholar] [CrossRef]
- Wong, M. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Bradshaw, A. Restoration of mined lands—Using natural processes. Ecol. Eng. 1997, 8, 255–269. [Google Scholar] [CrossRef]
- Martínez-Garza, C.; Howe, H.F. Restoring tropical diversity: Beating the time tax on species loss. J. Appl. Ecol. 2003, 40, 423–429. [Google Scholar] [CrossRef]
- Lacerda, L.D.; De Souza, M.; Ribeiro, M.G. The effects of land use change on mercury distribution in soils of Alta Floresta, Southern Amazon. Environ. Pollut. 2004, 129, 247–255. [Google Scholar] [CrossRef]
- Guedron, S. Impact de l ’Exploitation Minière en Guyane Française Sur les Flux de Mercure Vers les Écosystèmes Aquatiques, Grenoble; Université Joseph Fourier: Grenoble, France, 2008. [Google Scholar]
- Lombardi, A.T.; Garcia, O. Biological leaching of Mn, Al, Zn, Cu and Ti in an anaerobic sewage sludge effectuated by Thiobacillus ferrooxidans and its effect on metal partitioning. Water Res. 2002, 36, 3193–3202. [Google Scholar] [CrossRef]
- Ryu, H.W.; Moon, H.S.; Lee, E.Y.; Cho, K.S.; Choi, H. Leaching characteristics of heavy metals from sewage sludge by Acidithiobacillus thiooxidans MET. J. Environ. Qual. 1999, 32, 751–759. [Google Scholar] [CrossRef]
- Lovley, D.R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Rev. 1991, 55, 259–287. [Google Scholar] [CrossRef] [PubMed]
- Bousserrhine, N.; Gasser, U.G.; Jeanroy, E.; Berthelin, J. Bacterial and chemical reductive dissolution of Mn-, Co-, Cr-, and Al-substituted goethites. Geomicrobiol. J. 1999, 16, 245–258. [Google Scholar] [CrossRef]
- Schippers, A.; Sand, W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl. Environ. Microbiol. 1999, 65, 319–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledin, M. Accumulation of metals by microorganisms—Processes and importance for soil systems. Earth Sci. Rev. 2000, 51, 1–31. [Google Scholar] [CrossRef]
- Compeau, G.C.; Bartha, R. Sulfate-reducing bacteria: Principal methylators of mercury in anoxic estuarine sediment. Appl. Environ. Microbiol. 1985, 50, 498–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmour, C.C.; Henry, E.A.; Mitchell, R. Sulfate stimulation of mercury methylation in freshwater sediments. Environ. Sci. Technol. 1992, 26, 2281–2287. [Google Scholar] [CrossRef]
- Bousserrhine, N. Etude de Paramètres de la Réduction Bactérienne du fer et Application à la Déferrification de Minéraux Industriels; Université Nancy: Nancy, France, 1995. [Google Scholar]
- Roulet, M.; Lucotte, M. Geochemistry of mercury in pristine and flooded ferralitic soils of a tropical rain forest in French Guiana, South America. Water Air Soil Pollut. 1995, 80, 1079–1088. [Google Scholar] [CrossRef]
- Roulet, M.; Lucotte, M.; Saint-Aubin, A.; Tran, S.; Rhéault, I.; Farella, N.; De Jesus Da Silva, E.; Dezencourt, J.; Sousa Passos, C.J.; Santos Soares, G.; et al. The geochemistry of mercury in central Amazonian soils developed on the Alter-do-Chao formation of the lower Tapajos River Valley, Para state, Brazil. Sci. Total Environ. 1998, 223, 1–24. [Google Scholar] [CrossRef]
- Harris-Hellal, J.; Grimaldi, M.; Garnier-Zarli, E.; Bousserrhine, N. Mercury mobilization by chemical and microbial iron oxide reduction in soils of French Guyana. Biogeochemistry 2011, 103, 223–234. [Google Scholar] [CrossRef]
- Cooper, D.C.; Picardal, F.F.; Coby, A.J. Interactions between microbial iron reduction and metal geochemistry: Effect of redox cycling on transition metal speciation in iron bearing sediments. Environ. Sci. Technol. 2006, 40, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Schöner, A.; Dienemann, H.; Sauter, M. Release of coprecipitated uranium from iron oxides. J. Radioanal. Nucl. Chem. 2005, 267, 21–27. [Google Scholar] [CrossRef]
- Gounou, C.; Bousserrhine, N.; Varrault, G.; Mouchel, J.M. Influence of the iron-reducing bacteria on the release of heavy metals in anaerobic river sediment. Water Air Soil Pollut. 2010, 212, 123–129. [Google Scholar] [CrossRef]
- Lovley, D.R. Dissimilatory Metal Reduction. Annu. Rev. Microbiol. 1993, 47, 263–290. [Google Scholar] [CrossRef]
- Harris-Hellal, J. Etude des Interactions Entre sols–Mercure–Composante Microbiologique en Guyane Française; Université Paris Est: Paris, France, 2008. [Google Scholar]
- Alloway, B. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Springer: Cham, Switzerland, 2012; ISBN 9789400744691. [Google Scholar]
- Do Valle, C.M.; Santana, G.P.; Augusti, R.; Egreja Filho, F.B.; Windmöller, C.C. Speciation and quantification of mercury in Oxisol, Ultisol, and Spodosol from Amazon (Manaus, Brazil). Chemosphere 2005, 58, 779–792. [Google Scholar] [CrossRef]
- Fritsch, E.; Herbillon, A.J.; Do Nascimento, N.R.; Grimaldi, M.; Melfi, A.J. From Plinthic Acrisols to Plinthosols and Gleysols: Iron and groundwater dynamics in the tertiary sediments of the upper Amazon basin. Eur. J. Soil Sci. 2007, 58, 989–1006. [Google Scholar] [CrossRef]
- Le Roux, C. La réhabilitation des mines et carrières à ciel ouvert: Restauration des sites miniers. Bois For. Trop. 2002, 272, 5–19. [Google Scholar]
- Loubry, D. Livret Technique Pour la Conduite de la Revégétalisation sur les Surfaces Minières Alluvionanaires de Guyane. Available online: http://www.documentation.ird.fr/hor/fdi:010029513 (accessed on 22 September 2017).
- Barret, J. Illustrated Atlas of French Guyana; French Guy: Cayenne, France, 2004. [Google Scholar]
- Couic, E.; Alphonse, V.; Livet, A.; Giusti-Miller, S.; Bousserrhine, N. Influence of ecological restoration on mercury mobility and microbial activities on former guyanese mining sites. Appl. Sci. 2021, 11, 2231. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Jackson, M.L.; Barak, P. Soil Chemical Analysis: Advanced Course: A Manual of Methods Useful for Instruction and Research in Soil Chemistry, Physical Chemistry of Soils, Soil Fertility and Soil Genesis; Parallel Press, University of Wisconsin-Madison Libraries: Madison, WI, USA, 2005; ISBN 1893311473. [Google Scholar]
- Guédron, S.; Grimaldi, C.; Chauvel, C.; Spadini, L.; Grimaldi, M. Weathering versus atmospheric contributions to mercury concentrations in French Guiana soils. Appl. Geochem. 2006, 21, 2010–2022. [Google Scholar] [CrossRef]
- Guedron, S.; Grangeon, S.; Lanson, B.; Grimaldi, M. Mercury speciation in a tropical soil association; Consequence of gold mining on Hg distribution in French Guiana. Geoderma 2009, 153, 331–346. [Google Scholar] [CrossRef] [Green Version]
- Bousserrhine, N.; Gasser, U.; Jeanroy, E.; Berthelin, J. Effect of aluminium substitution on ferri-reducing bacterial activity and dissolution of goethites. C. R. l’Acad. Sci. Ser. IIA Sci. TerrePlanetes 1998, 326, 617–624. [Google Scholar] [CrossRef]
- Grimaldi, M.; Gaudet, J.P.; Grimaldi, C.; Melieres, M.A.; Spadini, L. Sources, Stocks et Transferts dans les sols et Sédiments. Programme Recherche. Mercur. Guyane Française. Rapp. Final. Partie I Région St Elie Réservoir Petit Saut; CNRS, Ed.; CNRS-PEVS: Paris, France, 2001. [Google Scholar]
- Anderson, A. The Biochemistry of Mercury in the Environment. In Mercury in Soils; Elsevier: Amsterdam, The Netherlands, 1979; pp. 79–112. ISBN 0444801103. [Google Scholar]
- Da Silva, E.; Nahmani, J.; Lapied, E.; Alphonse, V.; Garnier-Zarli, E.; Bousserrhine, N. Toxicity of mercury to the earthworm Pontoscolex corethrurus in a tropical soil of French Guiana. Appl. Soil Ecol. 2016, 104, 79–84. [Google Scholar] [CrossRef]
- Schimann, H.; Joffre, R.; Roggy, J.C.; Lensi, R.; Domenach, A.M. Evaluation of the recovery of microbial functions during soil restoration using near-infrared spectroscopy. Appl. Soil Ecol. 2007, 37, 223–232. [Google Scholar] [CrossRef]
- Schuster, E. The behavior of mercury in the soil with special emphasis on complexation and adsorption processes—A review of the literature. Water Air Soil Pollut. 1991, 56, 667–680. [Google Scholar] [CrossRef]
- Skyllberg, U.; Xia, K.; Bloom, P.R.; Nater, E.A.; Bleam, W.F. Binding of Mercury(II) to Reduced Sulfur in Soil Organic Matter along Upland-Peat Soil Transects. J. Environ. Qual. 2000, 29, 855. [Google Scholar] [CrossRef]
- Couic, E.; Grimaldi, M.; Alphonse, V.; Balland-Bolou-Bi, C.; Livet, A.; Giusti-Miller, S.; Sarrazin, M.; Bousserrhine, N. Mercury behaviour and C, N, and P biogeochemical cycles during ecological restoration processes of old mining sites in French Guiana. Environ. Sci. Process. Impacts 2018, 20, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Schimann, H.; Petit-Jean, C.; Guitet, S.; Reis, T.; Domenach, A.M.; Roggy, J.C. Microbial bioindicators of soil functioning after disturbance: The case of gold mining in tropical rainforests of French Guiana. Ecol. Indic. 2012, 20, 34–41. [Google Scholar] [CrossRef]
- Valentim dos Santos, J.; Varón-López, M.; Fonsêca Sousa Soares, C.R.; Lopes Leal, P.; Siqueira, J.O.; de Souza Moreira, F.M. Biological attributes of rehabilitated soils contaminated with heavy metals. Environ. Sci. Pollut. Res. 2016, 23, 6735–6748. [Google Scholar] [CrossRef]
- Lovley, D.R. Organic matter mineralization with the reduction of ferric iron: A review. Geomicrobiol. J. 1987, 5, 375–399. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.; Hu, Y.; Zhou, L.; Zheng, B. Growth characteristics of thermophile sulfate-reducing bacteria and its effect on carbon steel. Mater. Corros. 2009, 60, 218–224. [Google Scholar] [CrossRef]
- Martin, S. Precipitation and Dissolution of Iron and Manganese Oxides. In Environmental Catalysis; CRC Press: Boca Raton, FL, USA, 2005; pp. 61–82. [Google Scholar]
- Rose, S.; Ghazi, A.M. Release of sorbed sulfate from iron oxyhydroxides precipitated from acid mine drainage associated with coal mining. Environ. Sci. Technol. 1997, 31, 2136–2140. [Google Scholar] [CrossRef]
- Roden, E.E.; Lovley, D.R. Dissimilatory Fe(III) reduction by the marine microorganism Desulfuromonas acetoxidans. Appl. Environ. Microbiol. 1993, 59, 734–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blodau, C. A review of acidity generation and consumption in acidic coal mine lakes and their watersheds. Sci. Total Environ. 2006, 369, 307–332. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Plugge, C.M.; de Bok, F.A.M.; van Houten, B.H.G.W.; Lens, P.; Dijkman, H.; Weijma, J. Metabolic interactions in methanogenic and sulfate-reducing bioreactors. Water Sci. Technol. 2005, S2, 13–20. [Google Scholar] [CrossRef]
- Colleran, E.; Finnegan, S.; Lens, P. Anaerobic treatment of sulfate-containing waste streams. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 1995, 67, 29–46. [Google Scholar] [CrossRef]
- Widdel, F. Microbiology and ecology of sulfate-and sulfur-reducing bacteria. Zender AJB Biol. Anaerob. Microorg. NY 1988, 469–585. [Google Scholar] [CrossRef]
- Hao, O.J.; Chen, J.M.; Huang, L.; Buglass, R.L. Sulfate-reducing bacteria. Crit. Rev. Environ. Sci. Technol. 1996, 26, 155–187. [Google Scholar] [CrossRef]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Holmer, M.; Storkholm, P. Sulphate reduction and sulphur cycling in lake sediments: A review. Freshw. Biol. 2001, 46, 431–451. [Google Scholar] [CrossRef]
- Koschorreck, M. Microbial sulphate reduction at a low pH. FEMS Microbiol. Ecol. 2008, 64, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Hedrick, D.B.; Lovley, D.R.; White, D.C.; Pye, K. Reduction of Fe(III) in sediments by sulphate-reducing bacteria. Nature 1993, 361, 436–438. [Google Scholar] [CrossRef]
- Gabriel, M.C.; Williamson, D.G. Principal biogeochemical factors affecting the speciation and transport of mercury through the terrestrial environment. Environ. Geochem. Health 2004, 26, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Stemmler, S.J.; Berthelin, J. Microbial activity as a major factor in the mobilization of iron in the humid tropics. Eur. J. Soil Sci. 2003, 54, 725–733. [Google Scholar] [CrossRef]
- Wasserman, J.C.; Fundação, S.S.H.; Cruz, O.; Wasserman, M. Biogeochemistry of Mercury in the Amazonian Environment Mercury Exposure in Yanomami Indigenous Communities in the sate of Roraima View project Fate of nanoparticles in the environment View project. Ambio A J. Hum. Environ. 2003, 32, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A. Review of characteristics of mercury speciation and mobility from areas of mercury mining in semi-arid environments. Rev. Environ. Sci. Biotechnol. 2008, 7, 287–306. [Google Scholar] [CrossRef]
- Benoit, J.M.; Gilmour, C.C.; Mason, R.P. Aspects of bioavailability of mercury for methylation in pure cultures of Desulfobulbus propionicus (1pr3). Appl. Environ. Microbiol. 2001, 67, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Balland-Bolou-Bi, C.; Bolou-Bi, E.B.; Alphonse, V.; Giusti-Miller, S.; Jusselme, M.D.; Livet, A.; Grimaldi, M.; Bousserhine, N. Impact of microbial activity on the mobility of metallic elements (Fe, Al and Hg) in tropical soils. Geoderma 2019, 334, 146–154. [Google Scholar] [CrossRef]





| Sites | pH | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctot | TOC | MBC | Ntot | Ptot | Fe | Mn | Al | Hg | |||||
| g·kg−1 | g·kg−1 | mg·kg−1 | g·kg−1 | g·kg−1 | pH-H2O | g·kg−1 | g·kg−1 | g·kg−1 | µg·kg−1 | Clay% | Silt% | Sand% | |
| NR | 6.01 ± 0.32 a | 3.88 ± 0.04 a | 223 ± 10.8 a | 0.40 ± 0.03 a | 0.21 ± 0.04 ab | 5.26 ± 0.23 b | 65.15 ± 3.17 b | 0.52 ± 0.05 c | 53.88 ± 1.53 c | 0.23 ± 0.01 a | 46.4 ± 3.6 b | 41.5 ± 2.8 c | 3.3 ± 0.2 a |
| Lyc | 9.55 ± 0.73 b | 5.84 ± 0.35 b | 385.9 ± 21.5 b | 0.45 ± 0.01 a | 0.27 ± 0.07 b | 4.66 ± 0.07 a | 44.56 ± 0.77 a | 0.10 ± 0.003 a | 41.33 ± 1.77 b | 0.23 ± 0.07 a | 22.2 ± 5.1 a | 65.8 ± 3.2 d | 11.8 ± 1.2 b |
| Cli | 7.01 ± 0.83 a | 5.0 ± 0.4 b | 844.4 ± 14.03 c | 0.40 ± 0.04 a | 0.17 ± 0.02 a | 5.0 ± 0.03 b | 113.3 ± 4.2 c | 0.11 ± 0.004 a | 25.5 ± 2.15 a | 0.28 ± 0.03 a | 21.3 ± 1.6 a | 24.8 ± 3.7 a | 53.9 ± 2.5 d |
| Aca | 14.11 ± 1.2 c | 10.55 ± 0.62 c | 944 ± 79 c | 1.12 ± 0.07 b | 0.37 ± 0.12 c | 4.21 ± 0.07 a | 53.69 ± 4.15 a | 0.25 ± 0.05 b | 45.31 ± 6.51 b | 0.48 ± 0.04 b | 18.4 ± 2.5 a | 35.6 ± 3.9 b | 41.6 a ± 4.4 d |
| Mix | 22.88 ± 1.5 d | 15.58 ± 1.41 d | 1103 ± 41 d | 1.81 ± 0.10 c | 0.76 ± 0.15 d | 4.66 ± 0.11 a | 71.02 ± 1.37 b | 0.54 ± 0.03 c | 43.76 ± 1.21 b | 0.41 ± 0.05 b | 19.8 ± 3.8 a | 32.4 ± 4.9 b | 36.4 ± 5.9 c |
| Sites | Time | Sites × Time | Repeated-Measures ANOVA | ||
|---|---|---|---|---|---|
| Df | 4 | 5 | 20 | 5 | |
| pH | F-value | 114 * | 0.9 | 4.8 * | 0.61 |
| Heterotrophic respiration (µg CO2·µg Bio C−1) | 175 * | 513 | 35.6 | 14 * | |
| Dissolved Organic Carbon (mg DOC·L−1) | 215 * | 108 * | 105 * | 1.6 | |
| Sulfates (µg SO42−·L−1) | 59.9 * | 96.3 * | 4.4 * | 21 * | |
| Sulfides (µg S2−·L−1) | 305 * | 214 * | 58.5 * | 3.7 | |
| Soluble Fe (II) (mg Fe(II)·L−1) | 280 * | 174 * | 64.7 * | 2.8 | |
| Total soluble Fe (mg Fe·L−1) | 534 * | 285 * | 120 * | 2.65 | |
| Total soluble Al (mg Al·L−1) | 956 * | 864 * | 387 * | 3.6 | |
| Total soluble Mn (mg Mn·L−1) | 446 * | 375 * | 116 * | 3.2 | |
| Total soluble Hg (mg Hg·L−1) | 205 * | 66.5 * | 6.2 * | 11.3 * |
| pH | Sulfate | Sulfide | CO2 | DOC | Fe(II) | Fe | Mn | Al | Hg | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | 0.232 | −0.135 | 0.288 | 0.603 | 0.470 | 0.551 | 0.595 | 0.479 | −0.621 |
| Sulfate | 1 | 0.141 | 0.810 | 0.666 | 0.527 | 0.742 | 0.765 | 0.229 | 0.180 | |
| Sulfide | 1 | 0.197 | −0.209 | −0.117 | −0.148 | −0.186 | −0.070 | 0.102 | ||
| CO2 | 1 | 0.609 | 0.343 | 0.800 | 0.786 | 0.095 | −0.049 | |||
| DOC | 1 | 0.876 | 0.926 | 0.940 | 0.514 | −0.286 | ||||
| Fe(II) | 1 | 0.749 | 0.719 | 0.629 | −0.141 | |||||
| Fe | 1 | 0.977 | 0.409 | −0.218 | ||||||
| Mn | 1 | 0.384 | −0.252 | |||||||
| Al | 1 | −0.212 | ||||||||
| Hg | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couic, E.; Tribondeau, A.; Alphonse, V.; Livet, A.; Grimaldi, M.; Bousserrhine, N. The Impact of Ecological Restoration on Biogeochemical Cycling and Mercury Mobilization in Anoxic Conditions on Former Mining Sites in French Guiana. Microorganisms 2021, 9, 1702. https://doi.org/10.3390/microorganisms9081702
Couic E, Tribondeau A, Alphonse V, Livet A, Grimaldi M, Bousserrhine N. The Impact of Ecological Restoration on Biogeochemical Cycling and Mercury Mobilization in Anoxic Conditions on Former Mining Sites in French Guiana. Microorganisms. 2021; 9(8):1702. https://doi.org/10.3390/microorganisms9081702
Chicago/Turabian StyleCouic, Ewan, Alicia Tribondeau, Vanessa Alphonse, Alexandre Livet, Michel Grimaldi, and Noureddine Bousserrhine. 2021. "The Impact of Ecological Restoration on Biogeochemical Cycling and Mercury Mobilization in Anoxic Conditions on Former Mining Sites in French Guiana" Microorganisms 9, no. 8: 1702. https://doi.org/10.3390/microorganisms9081702