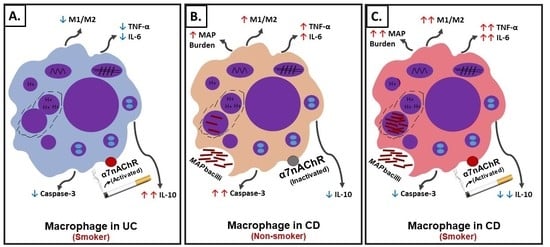
Mystery Solved: Why Smoke Extract Worsens Disease in Smokers with Crohn’s Disease and Not Ulcerative Colitis? Gut MAP!
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Conditions of THP-1 Macrophages
2.2. Infection and Treatment of THP-1 Macrophages
2.3. Measurement of Relative Gene Expression Using RT-PCR
2.4. Measurement of Cytokines and Cell Surface Markers
2.5. Bacterial Viability Assay
2.6. Measurement of Macrophage Apoptosis
2.7. Statistical Analysis
3. Results
3.1. Determination of Ingredient(s) in CS Extracts Active in Modulating Macrophages Response
3.2. Uninfected Cells
3.3. Infected Cells
3.3.1. In Absence of CS Extracts and Nicotine
3.3.2. In Presence of CS Extracts and Nicotine
3.4. Effect of Nicotine on Macrophages Response is Dose-Dependent
3.5. Effect of Nicotine on Macrophages Is Mediated through α7nAChRs
3.5.1. In Absence of Infection
3.5.2. During Infection
3.6. Pre-Conditioning Macrophages with Nicotine Protects against Infection-Induced Inflammation
Prior to Infection
3.7. Nicotine Increases MAP Burden in Macrophages and Decreases Cellular Apoptosis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.L.; Szamosi, T.; Lakatos, L. Smoking in inflammatory bowel diseases: Good, bad or ugly? World J. Gastroenterol. 2007, 13, 6134. [Google Scholar] [CrossRef] [PubMed]
- Bastida, G.; Beltrán, B. Ulcerative colitis in smokers, non-smokers and ex-smokers. World J. Gastroenterol. 2011, 17, 2740. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.L. Recent trends in the epidemiology of inflammatory bowel diseases: Up or down? World J. Gastroenterol. 2006, 12, 6102. [Google Scholar] [CrossRef]
- Harries, A.D.; Baird, A.; Rhodes, J. Non-smoking: A feature of ulcerative colitis. Br. Med. J. 1982, 284, 706. [Google Scholar] [CrossRef] [Green Version]
- Van der Heide, F.; Dijkstra, A.; Weersma, R.K.; Albersnagel, F.A.; van der Logt, E.M.; Faber, K.N.; Sluiter, W.J.; Kleibeuker, J.H.; Dijkstra, G. Effects of active and passive smoking on disease course of Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2009, 15, 1199–1207. [Google Scholar] [CrossRef]
- To, N.; Gracie, D.J.; Ford, A.C. Systematic review with meta-analysis: The adverse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment. Pharmacol. Ther. 2016, 43, 549–561. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Taneja, V.; Vassallo, R. Cigarette smoking and inflammation: Cellular and molecular mechanisms. J. Dent. Res. 2012, 91, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Boyko, E.J.; Koepsell, T.D.; Perera, D.R.; Inui, T.S. Risk of ulcerative colitis among former and current cigarette smokers. N. Engl. J. Med. 1987, 316, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Naser, S.A.; Ghobrial, G.; Romero, C.; Valentine, J.F. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet 2004, 364, 1039–1044. [Google Scholar] [CrossRef]
- Rashid, T.; Wilson, C.; Ebringer, A. The link between ankylosing spondylitis, Crohn’s disease, Klebsiella, and starch consumption. Clin. Dev. Immunol. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazareth, N.; Magro, F.; Machado, E.; Ribeiro, T.G.; Martinho, A.; Rodrigues, P.; Alves, R.; Macedo, G.N.; Gracio, D.; Coelho, R.; et al. Prevalence of Mycobacteriumavium subsp. paratuberculosis and Escherichia coli in blood samples from patients with inflammatory bowel disease. Med. Microbiol. Immunol. 2015, 204, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Rumsey, J.W.; Valentine, J.F.; Naser, S.A. Inhibition of phagosome maturation and survival of Mycobacterium avium subspecies paratuberculosis in polymorphonuclear leukocytes from Crohn’s disease patients. Med. Sci. Monit. 2006, 12, BR130-9. [Google Scholar] [CrossRef]
- Basu, J.; Shin, D.M.; Jo, E.K. Mycobacterial signaling through toll-like receptors. Front. Cell Infect. Microbiol. 2012, 2, 145. [Google Scholar] [CrossRef] [Green Version]
- Hallquist, N.; Hakki, A.; Wecker, L.; Friedman, H.; Pross, S. Differential effects of nicotine and aging on splenocyte proliferation and the production of Th1-versus Th2-type cytokines. Proc. Soc. Exp. Biol. Med. 2000, 224, 141–146. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Radek, K.A.; Elias, P.M.; Taupenot, L.; Mahata, S.K.; O’Connor, D.T.; Gallo, R.L. Neuroendocrine nicotinic receptor activation increases susceptibility to bacterial infections by suppressing antimicrobial peptide production. Cell Host Microbe 2010, 7, 277–289. [Google Scholar] [CrossRef] [Green Version]
- Blanchet, M.R.; Israël-Assayag, E.; Cormier, Y. Inhibitory effect of nicotine on experimental hypersensitivity pneumonitis in vivo and in vitro. Am. J. Respir. Crit. Care Med. 2004, 169, 903–909. [Google Scholar] [CrossRef] [Green Version]
- Russell, M.A.; Jarvis, M.; Iyer, R.; Feyerabend, C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. Br. Med. J. 1980, 280, 972–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourchez, J.; Parisse, S.; Sarry, G.; Perinel-Ragey, S.; Vergnon, J.M.; Clotagatide, A.; Prévôt, N. Impact of power level and refill liquid composition on the aerosol output and particle size distribution generated by a new-generation e-cigarette device. Aerosol Sci. Technol. 2018, 52, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Samet, J.M. Tobacco smoking: The leading cause of preventable disease worldwide. Thorac. Surg. Clin. 2013, 23, 103–112. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Kurokawa, M.; Ozaki, N.; Nara, K.; Atou, K.; Takada, E.; Kamochi, H.; Suzuki, N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-κB phosphorylation and nuclear factor-κB transcriptional activity through nicotinic acetylcholine receptor α7. Clin. Exp. Immunol. 2006, 146, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Mills, C. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qasem, A.; Naser, S.A. TNFα inhibitors exacerbate Mycobacterium paratuberculosis infection in tissue culture: A rationale for poor response of patients with Crohn’s disease to current approved therapy. BMJ Open Gastroenterol. 2018, 5, e000216. [Google Scholar] [CrossRef] [Green Version]
- Qasem, A.; Ramesh, S.; Naser, S.A. Genetic polymorphisms in tumour necrosis factor receptors (TNFRSF1A/1B) illustrate differential treatment response to TNFα inhibitors in patients with Crohn’s disease. BMJ Open Gastroenterol. 2019, 6, e000246. [Google Scholar] [CrossRef]
- Ji, M.; Zhang, Y.; Li, N.; Wang, C.; Xia, R.; Zhang, Z.; Wang, S.L. Nicotine component of cigarette smoke extract (cse) decreases the cytotoxicity of CSE in BEAS-2B cells stably expressing human cytochrome P450 2A13. Int. J. Environ. Res. Public Health 2017, 14. [Google Scholar] [CrossRef] [Green Version]
- Naser, S.A.; Sagramsingh, S.R.; Naser, A.S.; Thanigachalam, S. Mycobacterium avium subspecies paratuberculosis causes Crohn’s disease in some inflammatory bowel disease patients. World J. Gastroenterol. 2014, 20, 7403. [Google Scholar] [CrossRef]
- Bai, X.; Feldman, N.E.; Chmura, K.; Ovrutsky, A.R.; Su, W.L.; Griffin, L.; Pyeon, D.; McGibney, M.T.; Strand, M.J.; Numata, M.; et al. Inhibition of nuclear factor-kappa B activation decreases survival of Mycobacterium tuberculosis in human macrophages. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Crowley-Weber, C.L.; Dvorakova, K.; Crowley, C.; Bernstein, H.; Bernstein, C.; Garewal, H.; Payne, C.M. Nicotine increases oxidative stress, activates NF-κB and GRP78, induces apoptosis and sensitizes cells to genotoxic/xenobiotic stresses by a multiple stress inducer, deoxycholate: Relevance to colon carcinogenesis. Chem. Biol. Interact. 2003, 145, 53–66. [Google Scholar] [CrossRef]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011, 141, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Bishwakarma, R.; Kinney, W.H.; Honda, J.R.; Mya, J.; Strand, M.J.; Gangavelli, A.; Bai, X.; Ordway, D.J.; Iseman, M.D.; Chan, E.D. Epidemiologic link between tuberculosis and cigarette/biomass smoke exposure: Limitations despite the vast literature. Respirology 2015, 20, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Ordway, D.; Henao-Tamayo, M.; Bai, X.; Oberley-Deegan, R.; Shanley, C.; Orme, I.M.; Case, S.; Minor, M.; Ackart, D.; et al. Cigarette smoke increases susceptibility to tuberculosis—Evidence from in vivo and in vitro models. J. Infect. Dis. 2011, 203, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Parkes, G.C.; Whelan, K.; Lindsay, J.O. Smoking in inflammatory bowel disease: Impact on disease course and insights into the aetiology of its effect. J. Crohn’s Colitis 2014, 8, 717–725. [Google Scholar] [CrossRef]
- Lunney, P.C.; Leong, R.W. Ulcerative colitis, smoking and nicotine therapy. Aliment. Pharmacol. Ther. 2012, 36, 997–1008. [Google Scholar] [CrossRef]
- Ingram, J.R.; Thomas, G.A.; Rhodes, J.; Green, J.T.; Hawkes, N.D.; Swift, J.L.; Srivastava, E.D.; Evans, B.K.; Williams, G.T.; Newcombe, R.G.; et al. A randomized trial of nicotine enemas for active ulcerative colitis. Clin. Gastroenterol. Hepatol. 2005, 3, 1107–1114. [Google Scholar] [CrossRef]
- Bai, X.; Stitzel, J.A.; Bai, A.; Zambrano, C.A.; Phillips, M.; Marrack, P.; Chan, E.D. Nicotine impairs macrophage control of Mycobacterium tuberculosis. Am. J. Respir. Cell Mol. Biol. 2017, 57, 324–333. [Google Scholar] [CrossRef]
- Maor, I.; Rainis, T.; Lanir, A.; Lavy, A. Oxidative stress, inflammation and neutrophil superoxide release in patients with Crohn’s disease: Distinction between active and non-active disease. Dig. Dis. Sci. 2008, 53, 2208–2214. [Google Scholar] [CrossRef]
- Khan, M.Z.; Bhaskar, A.; Upadhyay, S.; Kumari, P.; Rajmani, R.S.; Jain, P.; Singh, A.; Kumar, D.; Bhavesh, N.S.; Nandicoori, V.K. Protein kinase G confers survival advantage to Mycobacterium tuberculosis during latency-like conditions. J. Biol. Chem. 2017, 292, 16093–16108. [Google Scholar] [CrossRef] [Green Version]
- Lakhan, S.E.; Kirchgessner, A. Anti-inflammatory effects of nicotine in obesity and ulcerative colitis. J. Transl. Med. 2011, 9, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, K.; Klein, T.W.; Friedman, H.; Yamamoto, Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J. Immunol. 2001, 167, 6518–6524. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Treatment Group | Viability Mean ± SD (%) | Fold Change in Viability Prior/Post Nicotine Treatment |
|---|---|---|
| K. pneumoniae ATCC 13883 | 3.7 ± 4.5 | |
| MAP UCF4 | 69.4 ± 5.2 | |
| N(4 µg/mL) + MAP UCF4 | 91.4 ± 5.5 | 1.31 |
| MAP UCF4 + N(4 µg/mL) | 80.79 ± 4.8 | 1.17 |
| MTB HR237 | 74.2 ± 3.1 | |
| N(4 µg/mL) + MTB HR237 | 92.5 ± 4.6 | 1.25 |
| MTB HR237 + N(4 µg/mL) | 85.2 ± 6.2 | 1.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlQasrawi, D.; Abdelli, L.S.; Naser, S.A. Mystery Solved: Why Smoke Extract Worsens Disease in Smokers with Crohn’s Disease and Not Ulcerative Colitis? Gut MAP! Microorganisms 2020, 8, 666. https://doi.org/10.3390/microorganisms8050666
AlQasrawi D, Abdelli LS, Naser SA. Mystery Solved: Why Smoke Extract Worsens Disease in Smokers with Crohn’s Disease and Not Ulcerative Colitis? Gut MAP! Microorganisms. 2020; 8(5):666. https://doi.org/10.3390/microorganisms8050666
Chicago/Turabian StyleAlQasrawi, Dania, Latifa S. Abdelli, and Saleh A. Naser. 2020. "Mystery Solved: Why Smoke Extract Worsens Disease in Smokers with Crohn’s Disease and Not Ulcerative Colitis? Gut MAP!" Microorganisms 8, no. 5: 666. https://doi.org/10.3390/microorganisms8050666