Kinetic Parameter Estimation and Mathematical Modelling of Lipase Catalysed Biodiesel Synthesis in a Microreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Chemicals
2.2. Methods
2.2.1. Lipase Assay
2.2.2. Emulsion Preparation
2.2.3. Measurement of Fatty Acid Methyl Esters (FAME) and Glycerol Concentrations
2.2.4. Biodiesel Synthesis in a Batch Reactor
2.2.5. Biodiesel Synthesis in Glass and PTFE Microreactors
2.2.6. Kinetic Parameter Estimation
2.2.7. Data Processing
2.3. Mathematical Modelling
2.3.1. Modelling of Biodiesel Transesterification
- Enzyme in aqueous phase (phase 1):
- Enzyme in oil phase (phase 2):
- Methanol in aqueous phase (phase 1):
- Methanol in oil phase (phase 2):
- Fatty acids in aqueous phase (phase 1):
- Fatty acids in oil phase (phase 2):
2.3.2. CFD Modelling
3. Results and Discussion
3.1. Biodiesel Production
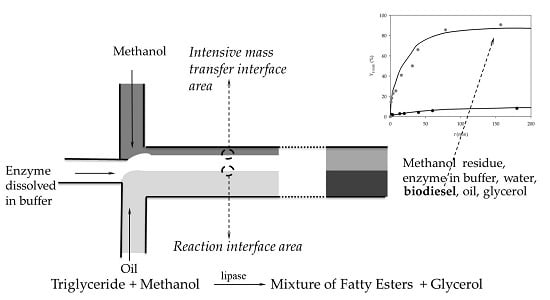
3.1.1. Two Inlets Strategy
3.2.2. Three Inlets Strategy—Glass Microreactor
3.2.3. Three Inlets Strategy—PTFE Microreactor
- The reaction occurs in the interphase area between phases.
- Even though triacylglycerols, diacylglycerols, monoacylglycerols, and free fatty acids are present in the mixture, they could be treated as a single constituent [38].
- Methanol is the main inhibitor of the enzyme, but due to the fact that the reaction is performed in a continuously operated tubular microreactor where methanol is constantly removed from the mixture, the inhibition effect was neglected [39].
- The limiting step of the reaction was considered to be hydrolysis, but according to literature, if the percentage of water in the process is between 2–20%, the reaction is shifted towards transesterification. In the present research, the water content was calculated to be below 8% (w/w), so the hydrolysis reaction was also neglected.
- It was assumed that the flow is laminar and parallel from the beginning of the microchannel.
3.2. Kinetic Parameter Estimation
3.3. Velocity Model of Multiphase Laminar Flow in a Microreactor
3.4. Model Validation
3.5. Process Optimization
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| A | Interface area (mm2) |
| c | Concentration (mmol/L) |
| DS/B | Diffusion coefficient (m2/s) |
| Fv | Volume force vector (N/m3) |
| Km | Michaelis–Menten constant (mmol/L) |
| L | Microchannel length (m) |
| MB | Molecular weight of solvent (g/mol) |
| p | Pressure (Pa) |
| T | Temperature (°C, K) |
| v | Linear velocity (m/s) |
| u | Velocity vector (m/s) |
| V | Reactor volume (μL) |
| V.A. | Volumetric activity (U/mL) |
| Vmax | Maximal reaction rate (U/mL) |
| VB | Molar volume of the solute (mL/mol) |
| VS | Molar volume of the solvent (mL/mol) |
| x | Microchannel length (mm) |
| y | Microchannel width (mm) |
| Y | FAME yield (%) |
| W | Half-width of microchannel (mm) |
| ρ | Solution density (kg/m3) |
| ηB | Dynamic viscosity (kg/(m⋅s)) |
| ξ | Dimensionless independent variables, x/W |
| τ | Residence time (s) |
| τD | Diffusion time (s) |
| υξ | X-directional velocity (m/s) |
| Φ | Flow rate (µL/min) |
| ψ | Dimensionless independent variables, y/W |
| aq | Aqueous phase |
| B | Buffer |
| E | Enzyme |
| FA | Fatty acids |
| i | Inlet |
| M | Methanol |
| MetOH | Methanol phase |
| S | Solute |
References
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Suurs, A.A.R.; Hekkert, M.P. Competition between first and second generation technologies: Lessons from the formation of a biofuels innovation system in the Netherlands. Energy 2009, 34, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Van Gerpen, J. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Biodiesel production technologies: Review. AIMS Energy 2017, 5, 425–457. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Palmeri, N.; Cavallaro, S. Industrial process technology for biodiesel production. In Biodiesel Science Technology Soil Oil; Woodhead Publishing: Sawston, UK, 2010; pp. 462–513. [Google Scholar]
- Issariyakul, T.; Dalai, A.K. Comparative kinetics of transesterification for biodiesel production from palm oil and mustard oil. Can. J. Chem. Eng. 2012, 90, 342–350. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sust. Energ. Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, S.; Gupta, M.N. Enzymatic transesterification for biodiesel production. Indian J. Biochem. Biophys. 2003, 40, 392–399. [Google Scholar] [PubMed]
- Mittelbach, M. Lipase catalyzed alcoholysis of sunflower oil. J. Am. Oil Chem. Soc. 1990, 67, 168–170. [Google Scholar] [CrossRef]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Teixeira, C.B.; Junior, J.V.M.; Macedo, A. Biocatalysis combined with physical technologies for development of a green biodiesel process. Renew. Sust. Energ. Rev. 2014, 33, 333–343. [Google Scholar] [CrossRef]
- Franjo, M.; Šalić, A.; Zelić, B. Microstructured devices for biodiesel production by transesterification. Biomass Convers. Biorefin. 2018, 8, 1005–1020. [Google Scholar] [CrossRef]
- Tonelli, F.; Evans, S.; Taticchi, P. Industrial sustainability: Challenges, perspectives, actions. Int. J. Bus. Inov. Res. 2013, 7, 143–163. [Google Scholar] [CrossRef]
- Budžaki, S.; Miljić, G.; Tišma, M.; Sundaram, S.; Hessel, V. Is there a future for enzymatic biodiesel industrial production in microreactors? Appl. Energy 2017, 201, 124–134. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, L.; Xu, N. Biodiesel synthesis in microreactors. Green Process. Synth. 2012, 1, 61–70. [Google Scholar] [CrossRef]
- Mazubert, A.; Poux, M.; Aubin, J. Intensified processes for FAME production from waste cooking oil: A technological review. Chem. Eng. J. 2013, 233, 201–223. [Google Scholar] [CrossRef] [Green Version]
- Madhawan, A.; Arora, A.; Das, J.; Kuila, A.; Sharma, V. Microreactor technology for biodiesel production: A review. Biomass Convers. Biorefin. 2018, 8, 485–496. [Google Scholar] [CrossRef]
- Šalić, A.; Tušek, A.J.; Sander, A.; Zelić, B. Lipase catalysed biodiesel synthesis with integrated glycerol separation in continuously operated microchips connected in series. New Biotechnol. 2018, 47, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Santana, H.S.; Silva, J.L.; Tortola, D.S.; Taranto, O.P. Transesterification of sunflower oil in microchannels with circular obstructions. Chin. J. Chem. Eng. 2018, 26, 852–863. [Google Scholar] [CrossRef]
- Mohammadi, F.; Rahimi, M.; Parvareh, A.; Feyzi, M. Stimulation of magnetic nanoparticles to intensify transesterification of soybean oil in micromixers for biodiesel production. Chem. Eng. Process. 2017, 122, 109–121. [Google Scholar] [CrossRef]
- Ulrich, T.; Borovinskaya, E.S.; Reschetilowski, W. Versuchsplanerische Untersuchung der Umesterung von Sojaöl mit Ethanol im Mikroreaktor. Chem. Ing. Tech. 2016, 88, 1455–1462. [Google Scholar] [CrossRef]
- Liu, J.; Chu, Y.; Cao, X.; Zhao, Y.; Xie, H.; Xue, S. Rapid transesterification of micro-amount of lipids from microalgae via a micro-mixer reactor. Biotechnol. Biofuels 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Lawal, A. Evaluation of Presulfided NiMo/γ-Al2O3 for Hydrodeoxygenation of Microalgae Oil to Produce Green Diesel. Energy Fuels 2015, 29, 262–272. [Google Scholar] [CrossRef]
- Mukherjeea, J.; Guptab, M.N. Dual bioimprinting of Thermomyces lanuginosus lipase for synthesis of biodiesel. Biotechnol. Rep. 2016, 10, 38–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuit, S.H.; Ong, Y.T.; Lee, K.T. Membrane technology as a promising alternative in biodiesel production: A review. Biotechnol. Adv. 2012, 30, 1364–1380. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Zhao, L.; Weatherley, L. Process intensification technologies in continuous biodiesel production. Chem. Eng. Process. 2010, 49, 323–330. [Google Scholar] [CrossRef]
- Kockmann, N.; Brand, O.; Fedder, G.K. Micro Process Engineering—Fundamentals, Modeling, Fabrication, and Applications, Advanced Micro and Nanosystems. In Handbook of Micro Reactors; Hessel, V., Renken, A., Schouten, J.C., Yoshida, J., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2006; pp. 394–402. [Google Scholar]
- Hessel, V.; Hardt, S.; Löwe, H. Chemical Micro Process Engineering: Fundamentals, Modelling and Reactions; Wiley-VCH Verlag: Weinheim, Germany, 2004. [Google Scholar]
- Budžaki, S.; Šalić, A.; Zelić, B.; Tišma, M. Enzyme-catalysed Biodiesel Production from Edible and Waste Cooking Oils. Chem. Biochem. Eng. Q. 2015, 29, 329–333. [Google Scholar] [CrossRef]
- Šalić, A.; Ivanković, M.; Ferk, E.; Zelić, B. ADH based NAD+ regeneration in a microreactor. J. Chem. Technol. Biotechnol. 2013, 8, 1721–1729. [Google Scholar] [CrossRef]
- Znidaršič-Plazl, P.; Plazl, I. Steroid extraction in a microchannel system—mathematical modelling and experiments. Lab. Chip 2007, 7, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Carr, P.W. Accuracy of Empirical Correlations for Estimating Diffusion Coefficients in Aqueous Organic Mixtures. Anal. Chem. 1997, 69, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E.; Carroad, P.A.; Bell, R.L. Estimation of diffusion coefficients of proteins. Biotechnol. Bioeng. 1980, 22, 947–955. [Google Scholar] [CrossRef]
- Yaws, C.L. Chemical Properties Handbook: Physical, Thermodynamics, Environmental Transport, Safety & Health Related Properties for Organic & Inorganic Chemical; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Zheng, Y.Y.; Guo, X.H.; Song, N.N.; Li, D.C. Thermophilic lipase from Thermomyces lanuginosus: Gene cloning, expression and characterization. J. Mol. Catal. B Enzym. 2011, 69, 127–132. [Google Scholar] [CrossRef]
- Akers, H.A.; Tuckler, V.E. The Molar Volume of a Solute. In Biochemistry and Molecular Biology Education; International Union of Biochemistry and Molecular Biology Inc.: Winnipeg, MB, Canada, 1985; pp. 136–137. [Google Scholar]
- Poe, S.L.; Cummings, M.A.; Haaf, M.P.; McQuade, D.T. Solving the clogging problem: Precipitate-forming reactions in flow. Angew. Chem. Ed. 2006, 45, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Nie, K.; Zhang, X.; Wang, M.; Deng, L.; Ye, X.; Wang, F.; Tan, T. Kinetic study on lipase-catalyzed biodiesel production from waste cooking oil. J. Mol. Catal. B Enzym. 2014, 99, 127–132. [Google Scholar] [CrossRef]
- Tušek, A.; Šalić, A.; Kurtanjek, Ž.; Zelić, B. Modelling and kinetic parameter estimation of alcohol dehydrogenase catalyzed hexanol oxidation in a microreactor. Eng. Life Sci. 2012, 12, 49–56. [Google Scholar] [CrossRef]
- Firdaus, M.Y.; Guo, Z.; Fedosov, S.N. Development of kinetic model for biodiesel production using liquid lipase as a biocatalyst, esterification step. Biochem. Eng. J. 2016, 105, 52–61. [Google Scholar] [CrossRef]
- Cheirsilp, B.; H-Kittikun, A.; Limkatanyu, S. Impact of transesterification mechanisms on the kinetic modeling of biodiesel production by immobilized lipase. Biochem. Eng. J. 2008, 42, 261–269. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, L.; Meng, X.; Xin, Z. Kinetics of transesterification of palm oil and dimethyl carbonate for biodiesel production at the catalysis of heterogeneous base catalyst. Bioresour. Technol. 2010, 101, 8144–8150. [Google Scholar]
- Veny, H.; Aroua, M.K.; Sulaiman, N.M.N. Kinetic study of lipase catalyzed transesterification of jatropha oil in circulated batch packed bed reactor. Chem. Eng. J. 2014, 237, 123–130. [Google Scholar] [CrossRef]
- Patnaik, P.R. On the dynamics of immobilized enzyme kinetics in a microreactor: A study of AP-catalyzed reactions. J. Biochem. Technol. 2011, 3, 285–288. [Google Scholar]








| Solute (S) | Solvent (B) | T (K) | VS (mL/mol) | VB (mL/mol) | MS (g/mol) | ηB (mPa·s) |
|---|---|---|---|---|---|---|
| Lipase | Water (buffer) | 313.15 | - | 18.069 [34] | 33,400 [35] | 0.654 |
| Methanol | Water (buffer) | 313.15 | 38.5 [36] | 18.069 [34] | 32.04 | 0.654 |
| Lipase | Methanol | 313.15 | - | 38.5 [36] | 33,400 [36] | 0.445 |
| Methanol | Methanol | 313.15 | 38.5 [36] | 38.5 [36] | 32.04 | 0.445 |
| Solute (S) | Solvent (B) | DS/B·10−9 (m2/s) | τD (s) |
|---|---|---|---|
| Lipase | Water (buffer) | 12.40 | - |
| Methanol | Water (buffer) | 2.09 | 7.48 |
| Lipase | Methanol | 18.22 | 85.7 |
| Methanol | Methanol | 1.71 | - |
| Effect of Residence Time | Effect of Enzyme Concentration | Effect of Oil to Methanol Ratio | |||
|---|---|---|---|---|---|
| τ (min) | YFAME (%) | γE (mg/mL) | YFAME (%) (τ = 180 min) | Oil:Methanol Molar Ratio | YFAME (%) (τ = 180 min) |
| 180 | 8.849 (8.18) | 0.1 | 8.849 (8.18) | 1:3.4 | 8.849 (8.18) |
| 270 | 9.729 | 0.2 | 9.505 | 1:17 | 40.938 |
| 360 | 10.245 | 0.3 | 10.282 | 1:34 | 87.179 (89.56) |
| 450 | 10.583 | - | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gojun, M.; Pustahija, L.; Jurinjak Tušek, A.; Šalić, A.; Valinger, D.; Zelić, B. Kinetic Parameter Estimation and Mathematical Modelling of Lipase Catalysed Biodiesel Synthesis in a Microreactor. Micromachines 2019, 10, 759. https://doi.org/10.3390/mi10110759
Gojun M, Pustahija L, Jurinjak Tušek A, Šalić A, Valinger D, Zelić B. Kinetic Parameter Estimation and Mathematical Modelling of Lipase Catalysed Biodiesel Synthesis in a Microreactor. Micromachines. 2019; 10(11):759. https://doi.org/10.3390/mi10110759
Chicago/Turabian StyleGojun, Martin, Lucija Pustahija, Ana Jurinjak Tušek, Anita Šalić, Davor Valinger, and Bruno Zelić. 2019. "Kinetic Parameter Estimation and Mathematical Modelling of Lipase Catalysed Biodiesel Synthesis in a Microreactor" Micromachines 10, no. 11: 759. https://doi.org/10.3390/mi10110759