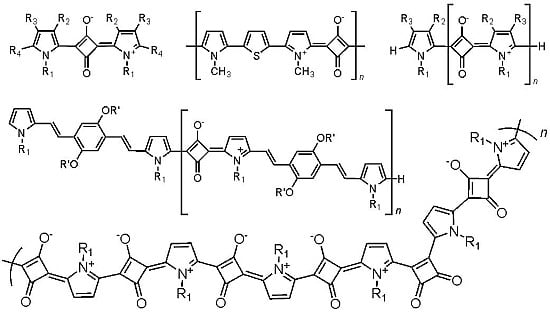
Pyrrolyl Squaraines–Fifty Golden Years
Abstract
:1. Introduction
2. Synthesis
2.1. The Beginning: The First Three Years


2.2. The Next Twenty Years: 1970–1990

2.3. Picking Up Pace: 1991–2000
2.4. The Pyrrolyl Squaraine Explosion: 2001–Current

2.5. Reaction Concentration and Time

2.6. Reaction Yields and Polymer Size

2.7. Check Your 1H NMR
3. Molecular Structure





4. Optical Properties
5. Electrical Properties

6. Magnetic Properties

7. Structural Properties
8. Toxicity
9. And Finally

10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Triebs, A.; Jacob, K. Cyclotrimethine dyes derived from squaric acid. Angew. Chem. Int. Ed. 1965, 4, 694. [Google Scholar] [CrossRef]
- Triebs, A.; Jacob, K. Cyclobutenederivate der pyrrolreihe. Liebigs Ann. Chem. 1966, 699, 153–167. [Google Scholar] [CrossRef]
- Triebs, A.; Jacob, K. Cyclobutenederivate der pyrrolreihe II. Liebigs Ann. Chem. 1968, 712, 123–137. [Google Scholar]
- Kazmaier, P.M.; Hamer, G.K.; Burt, R.A. Conformational isomerism in squaraines: Saturation transfer NMR studies on hydroxyl squaraines. Can. J. Chem. 1990, 68, 530–536. [Google Scholar] [CrossRef]
- Yu, L.P.; Chen, M.; Dalton, L.R.; Cao, X.F.; Jiang, J.P.; Hellworth, R.W. Synthesis and characterization of third order nonlinear optical materials. Mater. Res. Soc. Symp. Proc. 1990, 173, 607–612. [Google Scholar] [CrossRef]
- Patrick, B.; George, M.V.; Kamat, P.V.; Das, S.; Thomas, K.G. Photochemistry of squaraine dyes: Excited states and reduced and oxidized forms of 4-(4-acetyl-3,5-dimethylpyrrolium-2-ylidene)-2-(4-acetyl-3,5-dimethylpyrrol-2-yl)-3-oxocyclobut-1-en-1-olate. J. Chem. Soc. Faraday Trans. 1992, 88, 671–676. [Google Scholar] [CrossRef]
- Das, S.; Thomas, K.G.; Kamat, P.V.; George, M.V. Photosensitizing properties of squaraine dyes. Proc. Indian Acad. Sci. 1993, 105, 513–525. [Google Scholar] [CrossRef]
- Samoc, M.; Samoc, A.; Luther-Davies, B.; Woodruff, M. The concept of guiding light with light and negative third-order optical nonlinearities of organics. Pure Appl. Opt. 1996, 5, 681–687. [Google Scholar] [CrossRef]
- Geissler, U.; Lynch, D.E.; Rohde, N.; Hallensleben, M.L.; Walton, D.J. Poly(oligo(1-methylpyrrole))s and their squaraine-derivatives: An electrochemical and spectrochemical investigation. Synth. Met. 1997, 84, 171–172. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Chenthamarakshan, C.R.; Das, S.; George, M.V. Zwitterionic dye-based conducting polymers. Synthesis and optical properties of pyrrole-derived polysquaraines. Chem. Mater. 1997, 9, 644–646. [Google Scholar] [CrossRef]
- Lynch, D.E.; Geissler, U.; Peterson, I.R.; Floersheimer, M.; Terbrack, R.; Chi, L.F.; Fuchs, H.; Calos, N.J.; Wood, B.; Kennard, C.H.L.; et al. The synthesis and non-linear optical properties of (N-alkylpyrrol-2-yl)squaraine derivatives. Part 1. J. Chem. Soc. Perkin Trans. 2 1997. [Google Scholar] [CrossRef]
- Lynch, D.E.; Geissler, U.; Calos, N.J.; Wood, B.; Kinaev, N.N. Towards processible poly(pyrrol-2-ylsquaraines). Polym. Bull. 1998, 40, 373–380. [Google Scholar] [CrossRef]
- Ashwell, G.J.; Jefferies, G.; Green, A.; Skjonnemand, K.; Rees, N.D.; Bahra, G.S.; Brown, C.R. A comment upon the second-order non-linear optical properties of 2,4-bis(N-octadecylpyrrol-2-yl)squaraine. Thin Solid Films 1998, 327–329, 461–464. [Google Scholar] [CrossRef]
- Lynch, D.E.; Peterson, I.R.; Floersheimer, M.; Essing, D.; Chi, L.F.; Fuchs, H.; Calos, N.J.; Wood, B.; Kennard, C.H.L.; Langley, G.J. Synthesis and non-linear optical properties of (N-alkylpyrrol-2-yl)squaraine derivatives. Part 2. J. Chem. Soc. Perkin Trans. 2 1998. [Google Scholar] [CrossRef]
- Chenthamarakshan, C.R.; Ajayaghosh, A. Synthesis and properties of water-soluble oligomers containing pendant propanesulfonate moieties. Chem. Mater. 1998, 10, 1657–1663. [Google Scholar] [CrossRef]
- Chenthamarakshan, C.R.; Ajayaghosh, A. Enhanced sensitivity and selectivity in lithium ion recognition property of an oligomeric squaraine dye based fluorescent sensor. Tetrahedron Lett. 1998, 39, 1795–1798. [Google Scholar] [CrossRef]
- Chenthamarakshan, C.R.; Eldro, J.; Ajayaghosh, A. Synthesis and properties of alternating acceptor-donor copolymers of squaric acid with 1-decyl- and 3-dodecylpyrroles. Macromolecules 1999, 32, 251–257. [Google Scholar] [CrossRef]
- Chenthamarakshan, C.R.; Eldro, J.; Ajayaghosh, A. Squaraine dye based molecular wires containing flexible oxyethylene chains as sensors. Enhanced fluorescence response on Li+ recognition. Macromolecules 1999, 32, 5846–5851. [Google Scholar] [CrossRef]
- Keil, D.; Hartmann, H. Synthesis and characterization of a new class of unsymmetrical squaraine dyes. Dyes Pigment. 2001, 49, 161–179. [Google Scholar] [CrossRef]
- Lynch, D.E.; Geissler, U.; Byriel, K.A. An investigation into the electrical conduction properties of poly(oligo(1-methylpyrrol-2-yl)squaraine)s. Synth. Met. 2001, 124, 385–391. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Eldo, J. A novel approach toward low optical band gap polysquaraines. Org. Lett. 2001, 3, 2595–2598. [Google Scholar] [CrossRef] [PubMed]
- Eldo, J.; Ajayaghosh, A. New Low Band Gap Polymers: Control of optical and electronic properties in near infrared absorbing π-conjugated polysquaraines. Chem. Mater. 2002, 14, 410–418. [Google Scholar] [CrossRef]
- Buschel, M.; Ajayaghosh, A.; Arunkumar, E.; Daub, J. Redox-switchable squaraines with extended conjugation. Org. Lett. 2003, 5, 2975–2978. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R.; Motevalli, M.; Siu, J. Squaraines based on 2-arylpyrroles. Tetrahedron 2004, 60, 8913–8918. [Google Scholar] [CrossRef]
- Beverina, L.; Abbotto, A.; Landenna, M.; Cerminara, M.; Tubino, R.; Meinardi, F.; Bradamante, S.; Pagani, G.A. New π-extended water-soluble squaraines as singlet oxygen generators. Org. Lett. 2005, 7, 4257–4260. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.E.; Nawaz, Y.; Bostrom, T. Preparation of sub-micrometer silica shells using poly(1-methylpyrrol-2-ylsquaraine). Langmuir 2005, 21, 6572–6575. [Google Scholar] [CrossRef] [PubMed]
- Spicer, G.E.; Lynch, D.E.; Newman, A.P.; Coupe, S.J. The development of geotextiles incorporating slow-release phosphate beads for the maintenance of oil degrading bacteria in permeable pavements. Water Sci. Technol. 2006, 54, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, A.C.; de Siqueira, L.J.A.; Santos, P.S.; Temperini, M.L.A. Vibrational characterization of poly(1-methylpyrrole-co-squaric acid) and poly(1-dodecylpyrrole-co-squaric acid) by enhanced Raman spectroscopy. J. Raman Spectrosc. 2006, 37, 1346–1353. [Google Scholar] [CrossRef]
- Chung, S.J.; Zheng, S.; Odani, T.; Beverina, L.; Fu, J.; Padilha, L.A.; Biesso, A.; Hales, J.M.; Zhan, X.; Schmidt, K.; et al. Extended squaraine dyes with large two-photon absorption cross-sections. J. Am. Chem. Soc. 2006, 128, 14444–14445. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Huo, E.F.; Wu, Z.; Lu, Z.; Xie, M.G.; Jiang, Q. Novel poly(fluorine-alt-squaraine) derivatives having large coverage with solar spectrum. e-Polymers 2007, 077, 1–10. [Google Scholar]
- Huo, E.F.; Wu, J.Y.; Song, B.F.; Li, Y.; Jiang, Q.; Xie, M.G. Synthesis and characterization of novel low band-gap polymers containing squaraine units. Chin. Chem. Lett. 2007, 18, 1531–1534. [Google Scholar] [CrossRef]
- Beverina, L.; Crippa, M.; Landenna, M.; Ruffo, R.; Salice, P.; Silvestri, F.; Versari, S.; Villa, A.; Ciaffoni, L.; Collini, E.; et al. Assessment of water-soluble π-extended squaraines as one- and two-photon singlet oxygen photosensitizers: Design, synthesis, and characterization. J. Am. Chem. Soc. 2008, 130, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Beverina, L.; Crippa, M.; Salice, P.; Ruffo, R.; Ferrante, C.; Fortunati, I.; Signorini, R.; Mari, C.M.; Bozio, R.; Facchetti, A.; et al. Indolic Squaraines as Two-Photon Absorbing Dyes in the Visible Region: X-ray Structure, Electrochemical, and Nonlinear Optical Characterization. Chem. Mater. 2008, 20, 3242–3244. [Google Scholar] [CrossRef]
- Sreejith, S.; Divya, K.; Ajayaghosh, A. A near-infrared squaraine dye as a latent ratiometric fluorophore for the detection of aminothiol content in blood plasma. Angew. Chem. Int. Ed. 2008, 47, 7883–7887. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, F.; Irwin, M.D.; Beverina, L.; Facchetti, A.; Pagani, G.A.; Marks, T.J. Efficient squaraine-based solution processable bulk-heterojunction solar cells. J. Am. Chem. Soc. 2008, 130, 17640–17641. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.E. Particulate Materials. US Patent 0188611, 2008. [Google Scholar]
- Binda, M.; Agostinelli, T.; Caironi, M.; Natali, D.; Sampietro, M.; Beverina, L.; Ruffo, R.; Silvestri, F. Fast and air stable near-infrared organic detector based on squaraine dyes. Org. Electron. 2009, 10, 1314–1319. [Google Scholar] [CrossRef]
- Silvestri, F.; Lopez-Duarte, I.; Seitz, W.; Beverina, L.; Martinez-Diaz, M.V.; Marks, T.J.; Guldi, D.M.; Pagani, G.A.; Torres, T. A squaraine-phthalocyanine ensemble: Towards molecular panchromatic sensitizers in solar cells. Chem. Commun. 2009. [Google Scholar] [CrossRef] [PubMed]
- Beverina, L.; Ruffo, R.; Patriarca, G.; Angelis, F.D.; Roberto, D.; Righetto, S.; Ugo, R.; Pagani, G.A. Second harmonic generation in nonsymmetrical squaraines: Tuning of the directional charge transfer character in highly delocalized dyes. J. Mater. Chem. 2009, 19, 8190–8197. [Google Scholar] [CrossRef]
- Hasegawa, S.; Tian, M.; Ito, Y.; Hirokawa, K.; Watanabe, M.; Furuki, M. Black Color Material and Toner. US Patent 0240065, 2009. [Google Scholar]
- Zhang, W.; Wang, Z.; Tang, Y.S.; Xu, Z.G.; Li, Y.; Jiang, Q. Synthesis and properties of novel low band-gap polymers bearing squaraine units. Chin. Chem. Lett. 2010, 21, 245–248. [Google Scholar] [CrossRef]
- Lu, H.C.; Whang, W.T.; Cheng, B.M. Switchable structural modification accompanying altered optical properties of a zwitterionic polysquaraine. Chem. Phys. Lett. 2010, 500, 267–271. [Google Scholar] [CrossRef]
- Lu, H.C.; Whang, W.T.; Cheng, B.M. Effect of alkyl position of pyrrole on structures and properties of conjugated polysquaraines. Synth. Met. 2010, 160, 1002–1007. [Google Scholar] [CrossRef]
- Begum, S.; Jones, I.P.; Jiao, C.; Lynch, D.E.; Preece, J.A. Characterisation of hollow Russian doll microspheres. J. Mater. Sci. 2010, 45, 3697–3706. [Google Scholar] [CrossRef]
- Bagnis, D.; Beverina, L.; Huang, H.; Silvestri, F.; Yao, Y.; Yan, H.; Pagani, G.A.; Marks, T.J.; Facchetti, A. Marked alkyl- vs. alkenyl-substituted effects on squaraine dye solid-state structure, carrier mobility, and bulk-heterojunction solar cell efficiency. J. Am. Chem. Soc. 2010, 132, 4074–4075. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.J.; Chang, Y.L.; Hsiao, Y.N.; Chen, P.L.; Lin, S.H.; Whang, W.T.; Hsu, K.Y.; Tsai, M.H.; Tsang, W.Y. Co-doping with polysquaraine enhances the holographic optical data storage of PMMA/PQ photopolymers. J. Mod. Opt. 2011, 58, 1215–1219. [Google Scholar] [CrossRef]
- Lu, H.C.; Whang, W.T.; Cheng, B.M. Reversible isomerisation of a zwitterionic polysquaraine induced by a metal surface. J. Mater. Chem. 2011, 21, 2568–2576. [Google Scholar] [CrossRef]
- Paek, S.; Choi, H.; Kim, C.; Cho, N.; So, S.; Song, K.; Nazeeruddin, M.K.; Ko, J. Efficient and stable panchromatic squaraine dyes for dye-sensitized solar cells. Chem. Commun. 2011, 47, 2874–2876. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, W.Q.; Xiang, J.; Duan, X.M.; Zhan, X. A low-bandgap conjugated polymer based on squaraine with strong two-photon absorption. Macromolecules 2011, 44, 3759–3765. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, F.; Meng, K.; Wang, Z.; Xi, L.; Li, Y.; Jiang, Q. Synthesis and characterization of novel poly(aryleneethynylene)s derived from squaraines for photovoltaic applications. J. Mater. Sci. 2011, 46, 5363–5370. [Google Scholar] [CrossRef]
- Beverina, L.; Drees, M.; Facchetti, A.; Salamone, M.; Ruffo, R.; Pagani, G.A. Bulk heterojunction solar cells-tuning of the HOMO and LUMO energy levels of pyrrolic squaraine dyes. Eur. J. Org. Chem. 2011, 2011, 5555–5563. [Google Scholar] [CrossRef]
- Collini, E.; Carlotto, S.; Ferrante, C.; Bozio, R.; Polimeno, A.; Bloino, J.; Barone, V.; Ronchi, E.; Beverina, L.; Pagani, G.A. Multipolar symmetric squaraines with large two-photon absorption cross-sections in the NIR region. Phys. Chem. Chem. Phys. 2011, 13, 12087–12094. [Google Scholar] [CrossRef] [PubMed]
- So, S.; Choi, H.; Ko, H.M.; Kim, C.; Paek, S.; Cho, N.; Song, K.; Lee, J.K.; Ko, J. Novel unsymmetrical push-pull squaraine chromophores for solution processed small molecule bulk heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2012, 98, 224–232. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, F.; Meng, K.; Xi, L.; Wang, Z.; Li, Y.; Jiang, Q. Synthesis and properties of novel donor-acceptor copolymers based on thiophene and squaraine moieties. Polym. Bull. 2012, 68, 349–360. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Sheng, Y.; Wang, X.; Belfield, K.D. Near-infrared-emitting squaraine dyes with high 2PA cross-sections for multiphoton fluorescence imaging. Appl. Mater. Interfaces 2012, 4, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.C.; Chao, C.H.; Chen, C.H.; Wu, R.J.; Whang, W.T. Effect of ionic liquid on structure and properties of polysquaraines. Macromolecules 2012, 45, 3010–3016. [Google Scholar] [CrossRef]
- Beverina, L.; Ruffo, R.; Salamone, M.M.; Ronchi, E.; Binda, M.; Natali, D.; Sampietro, M. Panchromatic squaraine compounds for broad band light harvesting electronic devices. J. Mater. Chem. 2012, 22, 6704–6710. [Google Scholar] [CrossRef]
- Lynch, D.E.; Newman, D.M.; Wears, M.L.; Matelon, R.J. Novel magneto-optic behaviour from a polysquaraine. Synth. Met. 2013, 171, 15–22. [Google Scholar] [CrossRef]
- Lynch, D.E.; Ewington, J. The structure/property relationship in a series of pyrrolic polysquaraines. Synth. Met. 2013, 183, 40–49. [Google Scholar] [CrossRef]
- Courgneau, C.; Rusu, D.; Henneuse, C.; Ducruet, V.; Lacrampe, M.F.; Krawczak, P. Characterisation of low-odour emissive polylactide/cellulose fibre biocomposites for car interior. Express Polym. Lett. 2013, 7, 787–804. [Google Scholar] [CrossRef]
- Ho, M.C.; Chao, C.H.; Lo, A.Y.; Chen, C.H.; Wu, R.J.; Tsai, M.H.; Huang, Y.C.; Whang, W.-T. Significant improvement in the thermoelectric properties of zwitterionic polysquaraine composite films. Mater. Chem. Phys. 2013, 141, 920–928. [Google Scholar] [CrossRef]
- Huo, E.; Yang, D.; Zhang, Z.; Lu, Z.; Sun, H.; Xin, X.; Huang, Y.; Liu, Y.; Jiang, Q. Synthesis and characterization of 4-dodecyloxyphenyl and (4′-dodecyoxy-4-biphenyl)methylene-substituted bispyrrolylvinylthiophene-based polysquaraines having good solubility and very low bandgap for light absorption. J. Appl. Polym. Sci. 2013, 128, 1632–1639. [Google Scholar]
- Sassi, M.; Crippa, M.; Ruffo, R.; Turrisi, R.; Drees, M.; Pandey, U.K.; Termine, R.; Golemme, A.; Facchetti, A.; Beverina, L. Open circuit voltage tuning through molecular design in hydrazone end capped donors for bulk heterojunction solar cells. J. Mater. Chem. A 2013, 1, 2631–2638. [Google Scholar] [CrossRef]
- Lam, S.L.; Liu, X.; Zhao, F.; Lee, C.L.K.; Kwan, W.L. Manipulating open-circuit voltage in an organic photovoltaic device via a phenylalkyl side chain. Chem. Commun. 2013, 49, 4543–4545. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Lin, M.Y.; Chou, S.L.; Peng, Y.C.; Lo, J.I.; Shiu, H.W.; Chen, C.H.; Cheng, B.M. Linear and folded films of a zwitterionic polysquaraine. RSC Adv. 2013, 3, 21294–21297. [Google Scholar] [CrossRef]
- Alam, M.M.; Chattopadhyaya, M.; Chakrabarti, S.; Rizzo, A. One the origin of the very strong two-photon activity of squaraine dyes—A standard/damped response theory study. Phys. Chem. Chem. Phys. 2014, 16, 8030–8035. [Google Scholar] [CrossRef] [PubMed]
- Bellani, S.; Iacchetti, A.; Porro, M.; Beverina, L.; Antognazza, M.R.; Natali, D. Charge transport characterization in a squaraine-based photoconductor by means of admittance spectroscopy. Org. Electron. 2015, 22, 56–61. [Google Scholar] [CrossRef]
- Joseph, K.L.V.; Lim, J.; Anthonysamy, A.; Kim, H.; Choi, W.; Kim, J.K. Squaraine-sensitized composite of a reduced grapheme oxide/TiO2 photocatalyst: π-π stacking as a new method of dye anchoring. J. Mater. Chem. A 2015, 3, 232–239. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Yang, D.; Luo, Q.; Yang, L.; Huang, Y.; Zhao, S.; Lu, Z. Asymmetrical squaraines for high-performance small-molecule organic solar cells with a short circuit current of over 12 mA cm−2. Chem. Commun. 2015, 51, 6133–6136. [Google Scholar] [CrossRef] [PubMed]
- Ajayaghosh, A.; Anees, P. A Squaraine Based Fluorescent Probe and a Process for the Preparation Thereof. WO Patent 029050, 2015. [Google Scholar]
- Maahs, G.; Hegenberg, P. Syntheses and derivatives of squaric acid. Angew. Chem. Int. Ed. 1966, 5, 888–893. [Google Scholar] [CrossRef]
- Schmidt, A.H. Reaktionen von quadratsäure und quadratsäure-derivaten. Synthesis 1980, 12, 961–994. [Google Scholar] [CrossRef]
- Seitz, G.; Imming, P. Oxocarbons and pseudooxocarbons. Chem. Rev. 1992, 92, 1227–1260. [Google Scholar] [CrossRef]
- Ajayaghosh, A. Donor-acceptor type low band gap polymers: Polysquaraines and related systems. Chem. Soc. Rev. 2003, 32, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Ajayaghosh, A. Chemistry of squaraine-derived materials: Near-IR dyes, low band gap systems, and cation sensors. Acc. Chem. Res. 2005, 38, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, S.; Carol, P.; Chithra, P.; Ajayaghosh, A. Squaraine dyes: A mine of molecular materials. J. Mater. Chem. 2008, 18, 264–274. [Google Scholar] [CrossRef]
- McEwen, J.J.; Wallace, K.J. Squaraine dyes in molecular recognition and self-assembly. Chem. Commun. 2009. [Google Scholar] [CrossRef] [PubMed]
- Beverina, L.; Salice, P. Squaraine compounds: Tailored design and synthesis towards a variety of material science applications. Eur. J. Org. Chem. 2010, 2010, 1207–1225. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Li, Z.; Pei, J.; Tian, W. Solution processible D-A small molecules for bulk-heterojunction solar cells. Energy Environ. Sci. 2010, 3, 1427–1436. [Google Scholar] [CrossRef]
- Peng, H.; Chem, W.; Chem, Y.; Hakuna, L.; Strongin, R.; Wang, B. Thio reactive probes and chemosensors. Sensors 2012, 12, 15907–15946. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Wong, W.Y.; Han, L.H. Squaraine dyes for dye-sensitized solar cells: Recent advances and future challenges. Chem. Asian J. 2013, 8, 1706–1719. [Google Scholar] [CrossRef] [PubMed]
- Beverina, L.; Sassi, M. Twists and turns around a square: The many faces of squaraine chemistry. Synlett 2014, 25, 477–490. [Google Scholar] [CrossRef]
- Sprenger, H.E.; Ziegenbein, W. Condensation products of Squaric acid and tertiary aromatic amines. Angew. Chem. Int. Ed. 1966, 5, 894. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, H.E.; Ziegenbein, W. Das cyclobuten-diylium-kation, ein neuartiger chromophor aus quadratsäure. Angew. Chem. 1967, 79, 581–582. [Google Scholar] [CrossRef]
- Gassensmith, J.J.; Baumes, J.M.; Smith, B.D. Discovery and early development of squaraine rotaxanes. Chem. Commun. 2009. [Google Scholar] [CrossRef] [PubMed]
- Naarmann, H.; Köhler, G. Copolymere von Pyrrolen mit Quadratsäure, Verfahren zu Ihrer Herstellung Sowie Ihre Verwendung. German Patent 3246319, 1984. [Google Scholar]
- Vernitskaya, T.V.; Efimov, O.N. Polypyrrole: A conducting polymer; its synthesis, properties and Applications. Russ. Chem. Rev. 1997, 66, 443–457. [Google Scholar] [CrossRef]
- Marder, S.R.; Chen, C.T.; Cheng, L.T. Unsymmetrical Squaraines for Nonlinear Optical Materials. US Patent 5500156, 1996. [Google Scholar]
- West, R.M.; Cummins, W.J.; Nairne, R.J.D.; Bull, M.G. Squaraine Dyes. World Patent 58405, 2000. [Google Scholar]
- Cambridge Crystallographic Database Codes: LUSCUY, LUSDAF, SEZGIP and SEZGOV. Available online: http://www.ccdc.cam.ac.uk/pages/Home.aspx (accessed on 22 July 2015).
- Chen, Y.Y.; Hall, H.K. Polycondensation of Squaric acid with N-alkylcarbazoles. Polym. Bull. 1986, 16, 419–425. [Google Scholar] [CrossRef]
- Lynch, D.E.; Exilica Limited, The Technocentre, Puma Way, Coventry, UK. Unpublished Data. 2015.
- Ashwell, G.J.; Jefferies, G.; Hamilton, D.G.; Lynch, D.E.; Roberts, M.P.S.; Bahra, G.S.; Brown, C.R. Strong second-harmonic generation from centrosymmetric dyes. Nature 1995, 375, 385–388. [Google Scholar] [CrossRef]
- Havinga, E.E.; ten Hoeve, W.; Wynberg, H. A new class of small band gap organic polymer conductors. Polym. Bull. 1992, 29, 119–126. [Google Scholar] [CrossRef]
- De Leeuw, D.M.; Havinga, E.E.; Alberts, A.H.; Meekes, G.J.; Tol, A.J.W. N-type conductive polymer and method of preparing such a polymer. European Patent Application 0603939, 1992. [Google Scholar]
- Havinga, E.E.; ten Hoeve, W.; Wynberg, H. Alternate donor-acceptor small-band-gap semiconducting polymers: Polysquaraines and polycroconaines. Synth. Met. 1993, 55–57, 299–306. [Google Scholar] [CrossRef]
- Havinga, E.E.; Pomp, A.; ten Hoeve, W.; Wynberg, H. Water-soluble polysquaraines and polycroconaines. Synth. Met. 1995, 69, 581–582. [Google Scholar] [CrossRef]
- Pomfret, S.J.; Monkman, A.P.; Havinga, E.E. Electroabsorption measurements of polysquaraine. Synth. Met. 1996, 78, 285–288. [Google Scholar] [CrossRef]
- Tol, A.J.W. Using symmetry forbidden interactions to create small band gap polymers: Poly-aminosquaraine and related compounds. J. Chem. Phys. 1994, 100, 8463–8470. [Google Scholar] [CrossRef]
- Brocks, G. Ab initio electronic structure of a small band gap polymer: Poly-aminosquaraine. J. Chem. Phys. 1995, 102, 2522–2532. [Google Scholar] [CrossRef]
- Brocks, G.; Tol, A. Small band gap semiconducting polymers made from dye molecules: Polysquaraines. J. Phys. Chem. 1996, 100, 1838–1846. [Google Scholar] [CrossRef]
- Brocks, G.; Tol, A. A theoretical study of polysquaraines. Synth. Met. 1996, 76, 213–216. [Google Scholar] [CrossRef]
- Völker, S.F.; Dellermann, T.; Ceymann, H.; Holzapfel, M.; Lambert, C. Synthesis, electrochemical, and optical properties of low band gap homo- and copolymers based on squaraine dyes. J. Polym. Sci. Part A: Polym. Chem. 2014, 52, 890–911. [Google Scholar] [CrossRef]
- Tayi1, A.S.; Shveyd, A.K.; Sue, A.C.H.; Szarko, J.M.; Rolczynski, B.S.; Cao, D.; Kennedy, T.J.; Sarjeant, A.A.; Stern, C.L.; Paxton, W.F.; et al. Room-temperature ferroelectricity in supramolecular networks of charge-transfer complexes. Nature 2012, 488, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ago, H.; Yamabe, T. Design of ferromagnetic polymers involving organosilicon moieties. Synth. Met. 1995, 72, 225–229. [Google Scholar] [CrossRef]
- Gangopadhyay, P.; Koeckelberghs, G.; Persoons, A. Magneto-optic properties of regioregular polyalkylthiophenes. Chem. Mater. 2011, 23, 516–521. [Google Scholar] [CrossRef]
- Prostota, Y.; Kachkovsky, O.D.; Reis, L.V.; Santos, P.F. New unsymmetrical squaraine dyes derived from imidazo[1,5-a]pyridine. Dyes Pigment. 2013, 96, 554–562. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Z.M.; Qiu, Y.Q.; Pan, X.M.; Zhao, P.L.; Liao, Y.; Qin, C.S. Calculated UV-visible spectra and third-order nonlinear optical properties of heteroaromatic derivatives of stilbene inserted with 1,3-squaraine. Synth. Met. 2003, 137, 1525–1526. [Google Scholar] [CrossRef]
- Hartmann, H.; Keil, D.; Moschny, T. Synthesis and characterization of 1,3-bis-(2-dialkylamino-5-thienyl)-substituted squaraines—A novel class of intensely coloured panchromatic dyes. Dyes Pigment. 1991, 17, 19–27. [Google Scholar]
- Hartmann, H.; Keil, D.; Reichardt, C. Synthesis and spectroscopic characterization of new NIR absorbing (2-thienyl)- and (4-dialkylaminoaryl)-substituted croconic acid dyes. Liebigs Ann. Chem. 1993, 1993, 935–939. [Google Scholar]
- Hartmann, H.; Keil, D. Synthesis and characterization of 1,3-bis-(2-dialkylamino-5-thiazolyl)-substituted squaraines and their 2-(dialkylamino)thiazole precursors. Liebigs Ann. Chem. 1995, 1995, 979–984. [Google Scholar]
- Song, X.; Foley, J.W. A new water-soluble near-infrared croconium dye. Dyes Pigment. 2008, 78, 60–64. [Google Scholar] [CrossRef]
- Yagi, S.; Fujie, Y.; Hyodo, Y.; Nakazumi, H. Synthesis, structure, and complexation properties with transition metal cations of a novel methine-bridged bisquarylium dye. Dyes Pigment. 2002, 52, 245–252. [Google Scholar] [CrossRef]
- Yagi, S.; Murayama, S.; Hyodo, Y.; Fujie, Y.; Hirose, M.; Nakazumi, H. Synthesis and light absorption/emission properties of novel bis-squaraine dyes with extensively conjugated π-electron systems. J. Chem. Soc. Perkin Trans. 1 2002. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lynch, D.E. Pyrrolyl Squaraines–Fifty Golden Years. Metals 2015, 5, 1349-1370. https://doi.org/10.3390/met5031349
Lynch DE. Pyrrolyl Squaraines–Fifty Golden Years. Metals. 2015; 5(3):1349-1370. https://doi.org/10.3390/met5031349
Chicago/Turabian StyleLynch, Daniel E. 2015. "Pyrrolyl Squaraines–Fifty Golden Years" Metals 5, no. 3: 1349-1370. https://doi.org/10.3390/met5031349