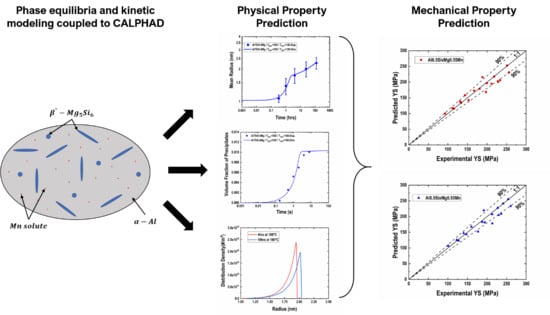
Modeling Precipitation Hardening and Yield Strength in Cast Al-Si-Mg-Mn Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Description
2.1.1. Nucleation Model
2.1.2. Growth Model
2.1.3. Yield Strength Model
Precipitation Hardening Model
Solid Solution Strengthening Model
2.2. Casting Trials and Heat Treatment Schedules
3. Results and Discussions
3.1. Model Implementation and Optimization
3.2. Yield Strength Prediction and Validation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryen, Ø.; Holmedal, B.; Nijs, O.; Nes, E.; Sjölander, E.; Ekström, H.-E. Strengthening mechanisms in solid solution aluminum alloys. Metall. Mater. Trans. A 2006, 37, 1999–2006. [Google Scholar] [CrossRef]
- Wagner, R.; Kampmann, R.; Voorhees, P.W. Homogeneous Second-Phase Precipitation. In Phase Transformations in Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2005; pp. 309–407. ISBN 9783527602643. [Google Scholar]
- Myhr, O.R.; Grong, Ø. Process modelling applied to 6082-T6 aluminium weldments—II. Applications of model. Acta Metall. Mater. 1991, 39, 2703–2708. [Google Scholar] [CrossRef]
- Myhr, O.R.; Grong, Ø.; Andersen, S.J. Modelling of the age hardening behaviour of Al–Mg–Si alloys. Acta Mater. 2001, 49, 65–75. [Google Scholar] [CrossRef]
- Du, Q.; Li, Y. An extension of the Kampmann–Wagner numerical model towards as-cast grain size prediction of multicomponent aluminum alloys. Acta Mater. 2014, 71, 380–389. [Google Scholar] [CrossRef]
- Rometsch, P.A.; Schaffer, G.B. An age hardening model for Al–7Si–Mg casting alloys. Mater. Sci. Eng. A 2002, 325, 424–434. [Google Scholar] [CrossRef]
- Sjölander, E.; Seifeddine, S.; Svensson, I.L. Modelling yield strength of heat treated Al–Si–Mg casting alloys. Int. J. Cast Met. Res. 2011, 24, 338–346. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Q.; Guo, H.; Xia, Z.; Wu, Q.; Liu, B. Modeling the precipitation kinetics and tensile properties in Al-7Si-Mg cast aluminum alloys. Mater. Sci. Eng. A 2017, 685, 403–416. [Google Scholar] [CrossRef]
- Gu, C.; Lu, Y.; Cinkilic, E.; Miao, J.; Klarner, A.; Yan, X.; Luo, A.A. Predicting grain structure in high pressure die casting of aluminum alloys: A coupled cellular automaton and process model. Comput. Mater. Sci. 2019, 161, 64–75. [Google Scholar] [CrossRef]
- Gu, C.; Lu, Y.; Ridgeway, C.D.; Cinkilic, E.; Luo, A.A. Three-dimensional cellular automaton simulation of coupled hydrogen porosity and microstructure during solidification of ternary aluminum alloys. Sci. Rep. 2019, 9, 13099. [Google Scholar] [CrossRef]
- Gu, C.; Ridgeway, C.D.; Cinkilic, E.; Lu, Y.; Luo, A.A. Predicting gas and shrinkage porosity in solidification microstructure: A coupled three-dimensional cellular automaton model. J. Mater. Sci. Technol. 2020, 49, 91–105. [Google Scholar] [CrossRef]
- Ridgeway, C.D.; Gu, C.; Ripplinger, K.; Detwiler, D.; Ji, M.; Soghrati, S.; Luo, A.A. Prediction of location specific mechanical properties of aluminum casting using a new CA-FEA (cellular automaton-finite element analysis) approach. Mater. Des. 2020, 194, 108929. [Google Scholar] [CrossRef]
- Du, Q.; Poole, W.J.; Wells, M.A. A mathematical model coupled to CALPHAD to predict precipitation kinetics for multicomponent aluminum alloys. Acta Mater. 2012, 60, 3830–3839. [Google Scholar] [CrossRef]
- Robson, J.D. Modelling the overlap of nucleation, growth and coarsening during precipitation. Acta Mater. 2004, 52, 4669–4676. [Google Scholar] [CrossRef]
- Robson, J.D.; Prangnell, P.B. Modelling Al3Zr dispersoid precipitation in multicomponent aluminium alloys. Mater. Sci. Eng. A 2003, 352, 240–250. [Google Scholar] [CrossRef]
- Seifeddine, S.; Wéssen, M.; Svensson, I.L. Use of simulation to predict microstructure and mechanical properties in an as-cast aluminium cylinder head comparison-with experiments. Metall. Sci. Tecnol. 2013, 24, 26–32. [Google Scholar]
- Zhang, C.; Cao, W.; Chen, S.L.; Zhu, J.; Zhang, F.; Luo, A.A.; Schmid-Fetzer, R. Precipitation Simulation of AZ91 Alloy. JOM 2014, 66, 389–396. [Google Scholar] [CrossRef]
- Robson, J.D. Modeling competitive continuous and discontinuous precipitation. Acta Mater. 2013, 61, 7781–7790. [Google Scholar] [CrossRef]
- Rettig, R.; Singer, R.F.F. Numerical modelling of precipitation of topologically close-packed phases in nickel-base superalloys. Acta Mater. 2011, 59, 317–327. [Google Scholar] [CrossRef]
- Rougier, L.; Jacot, A.; Gandin, C.-A.; Di Napoli, P.; Théry, P.-Y.; Ponsen, D.; Jaquet, V. Numerical simulation of precipitation in multicomponent Ni-base alloys. Acta Mater. 2013, 61, 6396–6405. [Google Scholar] [CrossRef]
- Bonvalet, M.; Philippe, T.; Sauvage, X.; Blavette, D. Modeling of precipitation kinetics in multicomponent systems: Application to model superalloys. Acta Mater. 2015, 100, 169–177. [Google Scholar] [CrossRef]
- Maugis, P.; Gouné, M. Kinetics of vanadium carbonitride precipitation in steel: A computer model. Acta Mater. 2005, 53, 3359–3367. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Enomoto, M. Numerical Simulation of Copper Precipitation during Aging in Deformed Fe-Cu Alloys. ISIJ Int. 2005, 45, 1335–1344. [Google Scholar] [CrossRef] [Green Version]
- Svoboda, J.; Fischer, F.D.; Fratzl, P.; Kozeschnik, E. Modelling of kinetics in multi-component multi-phase systems with spherical precipitates: I: Theory. Mater. Sci. Eng. A 2004, 385, 166–174. [Google Scholar] [CrossRef]
- Kozeschnik, E.; Svoboda, J.; Fratzl, P.; Fischer, F.D. Modelling of kinetics in multi-component multi-phase systems with spherical precipitates: II: Numerical solution and application. Mater. Sci. Eng. A 2004, 385, 157–165. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Q.; Guo, H.; Xia, Z.; Wu, Q.; Liu, B. Correlation of solidification microstructure refining scale, Mg composition and heat treatment conditions with mechanical properties in Al-7Si-Mg cast aluminum alloys. Mater. Sci. Eng. A 2017, 685, 391–402. [Google Scholar] [CrossRef]
- Holmedal, B.; Osmundsen, E.; Du, Q. Precipitation of Non-Spherical Particles in Aluminum Alloys Part I: Generalization of the Kampmann–Wagner Numerical Model. Metall. Mater. Trans. A 2016, 47, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.; Holmedal, B.; Friis, J.; Marioara, C.D. Precipitation of Non-spherical Particles in Aluminum Alloys Part II: Numerical Simulation and Experimental Characterization During Aging Treatment of an Al-Mg-Si Alloy. Metall. Mater. Trans. A 2016, 47, 589–599. [Google Scholar] [CrossRef]
- Myhr, O.R.; Grong, Ø.; Schäfer, C. An Extended Age-Hardening Model for Al-Mg-Si Alloys Incorporating the Room-Temperature Storage and Cold Deformation Process Stages. Metall. Mater. Trans. A 2015, 46, 6018–6039. [Google Scholar] [CrossRef]
- Bardel, D.; Perez, M.; Nelias, D.; Deschamps, A.; Hutchinson, C.R.; Maisonnette, D.; Chaise, T.; Garnier, J.; Bourlier, F. Coupled precipitation and yield strength modelling for non-isothermal treatments of a 6061 aluminium alloy. Acta Mater. 2014, 62, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Zhang, F.; Chen, S.-L.; Zhang, C.; Chang, Y.A. An integrated computational tool for precipitation simulation. JOM 2011, 63, 29–34. [Google Scholar] [CrossRef]
- Kelly, P.M. The effect of particle shape on dispersion hardening. Scr. Metall. 1972, 6, 647–656. [Google Scholar] [CrossRef]
- Xu, J.; Pan, Y.; Lu, T.; Bo, B. Synergistic effects of composition and heat treatment on microstructure and properties of vacuum die cast Al-Si-Mg-Mn alloys. China Foundry 2018, 15, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Perez, M.; Dumont, M.; Acevedo-Reyes, D. Implementation of classical nucleation and growth theories for precipitation. Acta Mater. 2008, 56, 2119–2132. [Google Scholar] [CrossRef]
- Campbell, C.E.; Boettinger, W.J.; Kattner, U.R. Development of a diffusion mobility database for Ni-base superalloys. Acta Mater. 2002, 50, 775–792. [Google Scholar] [CrossRef]
- Perez, M. Gibbs–Thomson effects in phase transformations. Scr. Mater. 2005, 52, 709–712. [Google Scholar] [CrossRef]
- Shahandeh, S.; Nategh, S. A computational thermodynamics approach to the Gibbs–Thomson effect. Mater. Sci. Eng. A 2007, 443, 178–184. [Google Scholar] [CrossRef]
- Myhr, O.R.; Hopperstad, O.S.; Børvik, T. A Combined Precipitation, Yield Stress, and Work Hardening Model for Al-Mg-Si Alloys Incorporating the Effects of Strain Rate and Temperature. Metall. Mater. Trans. A 2018, 49, 3592–3609. [Google Scholar] [CrossRef]
- Myhr, O.R.; Grong, Ø. Modelling of non-isothermal transformations in alloys containing a particle distribution. Acta Mater. 2000, 48, 1605–1615. [Google Scholar] [CrossRef]





| - | Weak Obstacle | Strong Obstacle |
|---|---|---|
| The resistance force of precipitates in a class | ||
| The average resistance force of obstacles | ||
| The average mean distance | ||
| The critical resolved shear stress (CRSS) | ||
| The precipitation hardening contribution | ||
| Alloy | Si | Mg | Fe | Mn | Zn | Sr | Ti | Cr |
|---|---|---|---|---|---|---|---|---|
| A1 | 6.66 | 0.184 | 0.123 | 0.552 | 0.005 | 0.0133 | 0.063 | 0.002 |
| A2 | 6.61 | 0.306 | 0.124 | 0.546 | 0.005 | 0.0132 | 0.062 | 0.002 |
| A3 | 6.58 | 0.441 | 0.123 | 0.538 | 0.005 | 0.0160 | 0.062 | 0.002 |
| B1 | 8.54 | 0.189 | 0.133 | 0.567 | 0.016 | 0.0144 | 0.064 | 0.003 |
| B2 | 8.54 | 0.316 | 0.137 | 0.558 | 0.017 | 0.0140 | 0.063 | 0.003 |
| B3 | 8.45 | 0.451 | 0.143 | 0.553 | 0.017 | 0.0137 | 0.062 | 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinkilic, E.; Yan, X.; Luo, A.A. Modeling Precipitation Hardening and Yield Strength in Cast Al-Si-Mg-Mn Alloys. Metals 2020, 10, 1356. https://doi.org/10.3390/met10101356
Cinkilic E, Yan X, Luo AA. Modeling Precipitation Hardening and Yield Strength in Cast Al-Si-Mg-Mn Alloys. Metals. 2020; 10(10):1356. https://doi.org/10.3390/met10101356
Chicago/Turabian StyleCinkilic, Emre, Xinyan Yan, and Alan A. Luo. 2020. "Modeling Precipitation Hardening and Yield Strength in Cast Al-Si-Mg-Mn Alloys" Metals 10, no. 10: 1356. https://doi.org/10.3390/met10101356