Early Findings after Implementation of Veno-Arteriovenous ECMO: A Multicenter European Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Study Outcomes
2.4. Statistical Procedures
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mosier, J.M.; Kelsey, M.; Raz, Y.; Gunnerson, K.J.; Meyer, R.; Hypes, C.D.; Malo, J.; Whitmore, S.P.; Spaite, D.W. Extracorporeal membrane oxygenation (ECMO) for critically ill adults in the emergency department: History, current applications, and future directions. Crit. Care 2015, 19, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betit, P. Technical Advances in the Field of ECMO. Respir. Care 2018, 63, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Danial, P.; Hajage, D.; Nguyen, L.S.; Mastroianni, C.; Demondion, P.; Schmidt, M.; Bouglé, A.; Amour, J.; Leprince, P.; Combes, A.; et al. Percutaneous versus surgical femoro-femoral veno-arterial ECMO: A propensity score matched study. Intensive Care Med. 2018, 44, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, L.; Lunz, D.; Philipp, A.; Lubnow, M.; Schmid, C. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel. 2015, 7, 320–326. [Google Scholar] [PubMed]
- Abrams, D.; Combes, A.; Brodie, D. Extracorporeal Membrane Oxygenation in Cardiopulmonary Disease in Adults. J. Am. Coll. Cardiol. 2014, 63, 2769–2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasseur, A.; Scolletta, S.; Lorusso, R.; Taccone, F.S. Hybrid extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10 (Suppl. 5), S707–S715. [Google Scholar] [CrossRef] [PubMed]
- Biscotti, M.; Lee, A.; Basner, R.C.; Agerstrand, C.; Abrams, D.; Brodie, D.; Bacchetta, M. Hybrid configurations via percutaneous access for extracorporeal membrane oxygenation: A single centre experience. ASAIO J. 2014, 60, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, S.W.; Kim, Y.U.; Kim, S.Y.; Kim, K.S.; Joo, S.J.; Lee, J.S. Application of veno-arterial-venous extracorporeal membrane oxygenation in differential hypoxia. Multidiscip. Respir. Med. 2014, 9, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ius, F.; Sommer, W.; Tudorache, I.; Avsar, M.; Siemeni, T.; Salman, J.; Puntigam, J.; Optenhoefel, J.; Greer, M.; Welte, T.; et al. Veno-veno-arterial extracorporeal membrane oxygenation for respiratory failure with severe haemodynamic impairment: Technique and early outcomes. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 761–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, N.L.; Coughlin, M.; Cooley, E.; Haft, J.W.; Hirschl, R.B.; Bartlett, R.H.; Mychaliska, G.B. The University of Michigan experience with venoveno-arterial hybrid mode of extracorporeal membrane oxygenation. ASAIO J. 2016, 62, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, F.; Emmert, M.Y.; Lachat, M.L.; Stocker, R.; Maggiorini, M.; Falk, V.; Wilhelm, M.J. Extracorporeal membrane oxygenation for acute respiratory distress syndrome: Is the configuration mode an important predictor for the outcome? Interact. Cardiovasc. Thorac. Surg. 2011, 12, 676–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, D.J.; Murray, J.; Czapran, A.Z.; Camporota, L.; Ioannou, N.; Meadows, C.I.S.; Sherren, P.B.; Daly, K.; Gooby, N.; Barrett, N. Veno-arterio-venous ECMO for septic cardiomyopathy: A single-centre experience. Perfusion 2018, 33 (Suppl. 1), 57–64. [Google Scholar] [CrossRef] [PubMed]
- Cakici, M.; Gumus, F.; Ozcinar, E.; Baran, C.; Bermede, O.; Inan, M.B.; Durdu, M.S.; Sirlak, M.; Akar, A.R. Controlled flow diversion in hybrid venoarterial-venous extracorporeal membrane oxygenation. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, H.J.; Jeon, D.; Kim, Y.S.; Cho, W.H.; Kim, D. Veno-veno-arterial extracorporeal membrane oxygenation treatment in patients with severe acute respiratory distress syndrome and septic shock. Crit. Care 2016, 20, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, L.; Sallisalmi, M.; Lindholm, J.A.; Lindfors, M.; Frenckner, B.; Broomé, M.; Broman, L.M. Differential hypoxemia during venoarterial extracorporeal membrane oxygenation. Perfusion 2019, 34 (Suppl. 1), 22–29. [Google Scholar] [CrossRef] [PubMed]


| All Patients (n = 32) | |||
|---|---|---|---|
| Age, Years | 51 (39–59) | ||
| Estimated Body Weight, kgs | 85 (77–96) | ||
| Male Sex, n (%) | 23 (72) | ||
| Days from admission to ECMO | 0 (0–2) | ||
| Days to V-AV ECMO | 2 (1–5) | ||
| Total days on ECMO | 9 (5–13) | ||
| ICU length of stay, days | 14 (9–23) | ||
| VV/VA ECMO | 16/16 | ||
| Drainage Cannula | |||
| femoral | 26 | ||
| jugular | 6 | ||
| Return Cannula | |||
| femoral artery | 16 | ||
| jugular vein | 9 | ||
| femoral vein | 6 | ||
| subclavian vein | 1 | ||
| Heart Disease, n (%) | 8 (25) | ||
| Diabetes, n (%) | 6 (19) | ||
| COPD/asthma, n (%) | 3 (9) | ||
| Neurological disease, n (%) | 1 (3) | ||
| Chronic hemodialysis, n (%) | - | ||
| Liver cirrhosis, n (%) | 3 (9) | ||
| Solid cancer, n (%) | 10 (31) | ||
| Hematological cancer, n (%) | 1 (3) | ||
| Immunosuppressive agents, n (%) | 10 (31) | ||
| CRRT, n (%) | 13 (41) | ||
| NMBAs, n (%) | 24 (75) | ||
| Dobutamine, n (%) | 6 (19) | ||
| iNO, n (%) | 8 (25) | ||
| SOFA score | 14 (12–16) | ||
| ICU Mortality, n (%) | 19 (59) | ||
| Before | After | p-Value | |
| Heart rate, bpm | 109 (94–121) | 91 (85–104) | <0.01 |
| Mean arterial pressure, mmHg | 71 (65–75) | 73 (70–75) | 0.38 |
| Central venous pressure, mmHg | 11 (8–13) | 11 (9–12) | 0.54 |
| Pulmonary arterial occlusive pressure, mmHg £ | 19 (17–21) | 20 (14–22) | 0.39 |
| Respiratory rate, rpm | 18 (13–22) | 14 (12–16) | <0.01 |
| Tidal volume, mL | 365 (217–452) | 280 (200–400) | 0.02 |
| PEEP, cmH2O | 10 (8–14) | 10 (8–13) | 0.73 |
| FiO2, % | 80 (60–100) | 50 (40–80) | <0.01 |
| SaO2, % | 90 (85–96) | 95 (92–98) | 0.03 |
| pH | 7.37 (7.28–7.42) | 7.38 (7.32–7.44) | 0.43 |
| PaCO2, mmHg | 40 (34–43) | 37 (35–42) | 0.29 |
| PaO2, mmHg | 63 (54–76) | 77 (64–92) | 0.02 |
| Temperature, °C | 36.6 (35.7–37.3) | 36.6 (36.0–37.1) | 0.74 |
| Lactate, mmol/L | 3.9 (2.3–7.1) | 2.8 (1.4–4.4) | 0.048 |
| Hemoglobin, g/dL | 10.1 (9.2–11.2) | 10.2 (9.5–11.6) | 0.81 |
| Creatinine, mg/dL | 1.3 (1.0–1.9) | 1.3 (1.0–1.9) | 0.58 |
| Norepinephrine dose, mcg/min # | 40 (15–71) | 21 (13–37) | <0.01 |
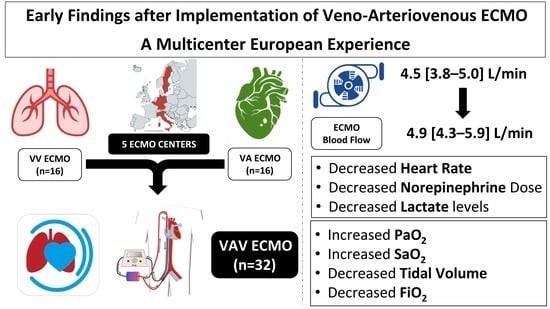
| ECMO blood flow, L/min | 4.5 (3.8–5.0) | 4.9 (4.3–5.9) | <0.01 |
| ECMO gas flow, L/min | 6 (5–8) | 6 (4–8) | 0.71 |
| ECMO FEO2, % | 100 (100–100) | 100 (100–100) | 0.99 |
| VA (n = 16) | VV (n = 16) | |
|---|---|---|
| Age, years | 49 (36–58) | 51 (41–59) |
| Male sex, n (%) | 13 (81) | 10 (63) |
| Days from admission to ECMO | 0 (0–2) | 0 (0–4) |
| Days to V-AV ECMO | 2 (1–8) | 1 (0–3) |
| Total days on ECMO | 9 (5–19) | 10 (6–12) |
| ICU length of stay, days | 14 (8–22) | 14 (9–25) |
| CRRT, n (%) | 7 (44) | 6 (38) |
| NMBAs, n (%) | 14 (88) | 10 (63) |
| Dobutamine, n (%) | 6 (19) | 1 (6) |
| iNO, n (%) | 7 (44) | 1 (6) * |
| SOFA score | 15 (13–16) | 13 (12–15) |
| ICU mortality, n (%) | 10 (62) | 9 (56) |
| Heart rate, bpm | 104 (91–117) | 120 (99–125) |
| Mean arterial pressure, mmHg | 73 (70–76) | 67 (60–75) |
| Central venous pressure, mmHg | 11 (8–13) | 11 (8–12) |
| Respiratory rate, rpm | 21 (15–25) | 15 (12–18) |
| Tidal volume, mL | 390 (269–458) | 328 (180–434) |
| PEEP, cmH2O | 12 (10–14) | 9 (7–13) |
| FiO2, % | 80 (68–100) | 90 (50–100) |
| SaO2, % | 90 (86–93) | 93 (79–96) |
| pH | 7.39 (7.33–7.44) | 7.34 (7.28–7.40) |
| PaCO2, mmHg | 38 (33–44) | 40 (34–43) |
| PaO2, mmHg | 62 (55–73) | 64 (50–91) |
| Temperature, °C | 36.6 (36.0–37.2) | 36.8 (35.5–37.4) |
| Lactate, mmol/L | 3.9 (2.4–5.3) | 4.3 (2.3–10.7) |
| Hemoglobin, g/dL | 10.2 (9.2–11.1) | 10.1 (9.3–11.7) |
| Norepinephrine dose, mcg/min * | 43 (23–77) (n = 15) | 33 (13–71) (n = 12) |
| ECMO Blood Flow, L/min | 3.9 (3.7–4.5) | 4.9 (4.4–5.5) |
| ECMO Gas Flow, L/min | 6 (4–8) | 7 (6–8) |
| ECMO FEO2, % | 100 (100–100) | 100 (100–100) |
| VA ECMO | Before (n = 16) | After (n = 16) | p-Value |
| Heart Rate, bpm | 104 (91–117) | 89 (84–103) | <0.01 |
| Mean Arterial Pressure, mmHg | 73 (70–76) | 72 (68–75) | 0.23 |
| Central Venous Pressure, mmHg | 11 (8–13) | 10 (8–12) | 0.18 |
| Respiratory Rate, rpm | 21 (15–25) | 15 (12–21) | 0.02 |
| Tidal Volume, mL | 390 (269–458) | 245 (200–315) | <0.01 |
| PEEP, cmH2O | 12 (10–14) | 11 (8–13) | 0.45 |
| FiO2, % | 80 (68–100) | 50 (43–78) | 0.03 |
| SaO2, % | 90 (86–93) | 97 (92–100) | <0.01 |
| pH | 7.39 (7.33–7.44) | 7.39 (7.33–7.47) | 0.49 |
| PaCO2, mmHg | 38 (33–44) | 37 (34–41) | 0.59 |
| PaO2, mmHg | 62 (55–73) | 75 (61–101) | <0.01 |
| Temperature, °C | 36.6 (36.0–37.2) | 36.7 (35.6–37.2) | 0.55 |
| Lactate, mmol/L | 3.9 (2.4–5.3) | 2.9 (1.2–4.1) | 0.28 |
| Hemoglobin, g/dL | 10.2 (9.2–11.1) | 10.1 (9.4–11.5) | 0.78 |
| Norepinephrine Dose, mcg/min | 43 (23–77) (n = 15) | 25 (9–61) (n = 15) | 0.16 |
| ECMO Blood Flow, L/min | 3.9 (3.7–4.5) | 4.9 (4.3–5.5) | <0.01 |
| ECMO Gas Flow, L/min | 6 (4–8) | 7 (4–8) | 0.21 |
| ECMO FEO2, % | 100 (100–100) | 100 (100–100) | 0.99 |
| VV ECMO | Before (n = 16) | After (n = 16) | p-Value |
| Heart Rate, bpm | 120 (99–125) | 92 (85–111) | <0.01 |
| Mean Arterial Pressure, mmHg | 67 (60–75) | 73 (70–80) | 0.17 |
| Central Venous Pressure, mmHg | 11 (8–12) | 9 (7–11) | 0.07 |
| Respiratory Rate, rpm | 15 (12–18) | 13 (9–20) | 0.02 |
| Tidal Volume, mL | 328 (180–434) | 305 (120–450) | 0.46 |
| PEEP, cmH2O | 9 (7–13) | 9 (8–13) | 0.83 |
| FiO2, % | 90 (50–100) | 60 (37–95) | 0.12 |
| SaO2, % | 93 (79–96) | 95 (88–98) | 0.13 |
| pH | 7.34 (7.28–7.40) | 7.37 (7.27–7.42) | 0.85 |
| PaCO2, mmHg | 40 (34–43) | 37 (35–43) | 0.28 |
| PaO2, mmHg | 64 (50–91) | 77 (66–92) | 0.47 |
| Temperature, °C | 36.8 (35.5–37.4) | 37.0 (36.3–37.3) | 0.67 |
| Lactate, mmol/L | 4.3 (2.3–10.7) | 2.8 (1.5–8.9) | 0.15 |
| Hemoglobin, g/dL | 10.1 (9.3–11.7) | 10.0 (9.2–11.3) | 0.87 |
| Norepinephrine Dose, mcg/min | 33 (13–71) (n = 12) | 21 (12–30) (n = 12) | 0.10 |
| ECMO Blood Flow, L/min | 4.9 (4.4–5.5) | 5.4 (4.3–6.2) | 0.049 |
| ECMO Gas Flow, L/min | 7 (6–8) | 6 (3–8) | 0.67 |
| ECMO FEO2, % | 100 (100–100) | 100 (100–100) | 0.99 |
| Study (REF) | Type | N | Adults (%) | Data (Years) | Initial ECMO (VA, VV, V-AV) | ECPR | Mortality |
|---|---|---|---|---|---|---|---|
| Werner [11] | R | 31 | 23 (74) | 14 | 2/31 (31%) 8/31 (26%) 11/31 (35%) | 9/23 (39%) | 14/23 (61%) |
| Biscotti [7] | R | 21 | 21 (100) | 2 | 8/21 (38%) 2/21 (10%) 11/21 (52%) | 7/21 (33%) | 12/21 (57%) |
| Ius [10] | R | 10 | 10 (100) | 3 | 9/10 (90%) 1/10 (10%) 0 | 0 | 5/10 (50%) |
| Stöhr [12] | P | 11 | 11 (100) | 3 | 3/11 (27%) 5/11 (45%) 3/11 (27%) | 0 | 3/11 (27%) |
| Vogel [13] | R | 12 | 12 (100) | 3 | 7/12 (67%) 0 0 | 5/12 (42%) | 3/12 (25%) |
| Cakici [14] | R | 12 | 12 (100) | 2 | 9/12 (75%) 0 0 | 2/12 (8%) | 4/12 (33%) |
| Yeo [15] | R | 8 | 8 (100) | 3 | 8/8 (100%) 0 0 | 0 | 4/8 (50%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blandino Ortiz, A.; Belliato, M.; Broman, L.M.; Lheureux, O.; Malfertheiner, M.V.; Xini, A.; Pappalardo, F.; Taccone, F.S. Early Findings after Implementation of Veno-Arteriovenous ECMO: A Multicenter European Experience. Membranes 2021, 11, 81. https://doi.org/10.3390/membranes11020081
Blandino Ortiz A, Belliato M, Broman LM, Lheureux O, Malfertheiner MV, Xini A, Pappalardo F, Taccone FS. Early Findings after Implementation of Veno-Arteriovenous ECMO: A Multicenter European Experience. Membranes. 2021; 11(2):81. https://doi.org/10.3390/membranes11020081
Chicago/Turabian StyleBlandino Ortiz, Aaron, Mirko Belliato, Lars Mikael Broman, Olivier Lheureux, Maximilian Valentin Malfertheiner, Angela Xini, Federico Pappalardo, and Fabio Silvio Taccone. 2021. "Early Findings after Implementation of Veno-Arteriovenous ECMO: A Multicenter European Experience" Membranes 11, no. 2: 81. https://doi.org/10.3390/membranes11020081