Formation of Microfiltration Membranes from PMP/PIB Blends: Effect of PIB Molecular Weight on Membrane Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Membrane Formation
2.2. Methods
3. Results and Discussion
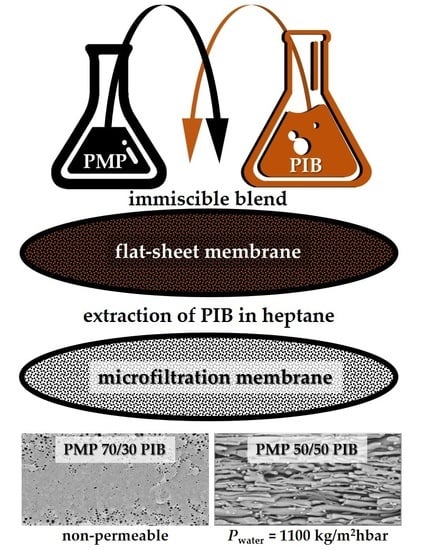
3.1. Effect of PIB Molecular Weight
3.2. Effect of PMP/PIB Ratio
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Strathmann, H.; Kock, K. The formation mechanism of phase inversion membranes. Desalination 1977, 21, 241–255. [Google Scholar] [CrossRef]
- Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Wienk, I.M.; Boom, R.M.; Beerlage, M.A.M.; Bulte, A.M.W.; Smolders, C.A.; Strathmann, H. Recent advances in the formation of phase inversion membranes made from amorphous or semi-crystalline polymers. J. Membr. Sci. 1996, 113, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Kimmerle, K.; Strathmann, H. Analysis of the structure-determining process of phase inversion membranes. Desalination 1990, 79, 283–302. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Anokhina, T.S.; Ignatenko, V.Y.; Brantseva, T.V.; Volkov, A.V.; Antonov, S.V. Diffusion and phase separation at the morphology formation of cellulose membranes by regeneration from N-methylmorpholine N-oxide solutions. Cellulose 2018, 25, 2515–2530. [Google Scholar] [CrossRef]
- Wilczynski, A.P.; Liu, C.H.; Hsiao, C.C. Mechanics of polymer craze. J. Appl. Phys. 1977, 48, 1149–1154. [Google Scholar] [CrossRef]
- Sadeghi, F.; Ajji, A.; Carreau, P.J. Analysis of microporous membranes obtained from polypropylene films by stretchin. J. Memb. Sci. 2007, 292, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Michaels, A.S.; Bixler, H.J.; Hopfenberg, H.B. Controllably crazed polystyrene: Morphology and permeability. J. Appl. Polym. Sci. 1968, 12, 991–1007. [Google Scholar] [CrossRef]
- Jacques, C.H.M.; Hopfenberg, H.B.; Stannett, V. The effect of orientation on the morphology and kinetics of solvent crazing in polystyrene. J. Appl. Polym. Sci. 1974, 18, 223–233. [Google Scholar] [CrossRef]
- He, D.; Susanto, H.; Ulbricht, M. Photo-irradiation for preparation, modification and stimulation of polymeric membrane. Progr. Polym. Sci. 2009, 34, 62–98. [Google Scholar] [CrossRef]
- Apel, P. Track etching technique in membrane technology. Radiat. Meas. 2001, 34, 559–566. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publisher: London, UK, 1997; p. 563. [Google Scholar]
- Matsuyama, H.; Okafuji, H.; Maki, T.; Teramoto, M.; Tsujioka, N. Membrane formation via thermally induced phase separation in polypropylene/polybutene/diluent system. J. Appl. Polym. Sci. 2002, 84, 1701–1708. [Google Scholar] [CrossRef]
- Esquirol, A.L.; Sarazin, P.; Virgilio, N. Tunable Porous Hydrogels from Cocontinuous Polymer Blends. Macromolecules 2014, 47, 3068–3075. [Google Scholar] [CrossRef]
- Zeng, M.; Fang, Z.; Xu, C. Novel method of preparing microporous membrane by selective dissolution of chitosan/polyethylene glycol blend membrane. J. Appl. Polym. Sci. 2004, 91, 2840–2847. [Google Scholar] [CrossRef]
- Zeng, M.; Fang, Z.; Xu, C. Effect of compatibility on the structure of the microporous membrane prepared by selective dissolution of chitosan/synthetic polymer blend membrane. J. Membr. Sci. 2004, 230, 175–181. [Google Scholar] [CrossRef]
- Trifkovic, M.; Hedegaard, A.; Huston, K.; Sheikhzadeh, M.; Macosko, C.W. Porous films via PE/PEO cocontinuous blends. Macromolecules 2012, 45, 6036–6044. [Google Scholar] [CrossRef]
- Anokhina, T.S.; Ilyin, S.O.; Ignatenko, V.Y.; Bakhtin, D.S.; Kostyuk, A.V.; Antonov, S.V.; Volkov, A.V. Formation of Porous Films with Hydrophobic Surface from a Blend of Polymers. Polym. Sci. Ser. A 2019, 61, 619–626. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Anokhina, T.S.; Ilyin, S.O.; Kostyuk, A.V.; Bakhtin, D.S.; Antonov, S.V.; Volkov, A.V. Fabrication of microfiltration membranes from polyisobutylene/polymethylpentene blends. Polym. Int. 2019. [Google Scholar] [CrossRef]
- Chandavasu, C.; Xanthos, M.; Sirkar, K.K.; Gogos, C.G. Fabrication of microporous polymeric membranes by melt processing of immiscible blends. J. Membr. Sci. 2003, 211, 167–175. [Google Scholar] [CrossRef]
- Femmer, T.; Kuehne, A.J.C.; Torres-Rendon, J.; Walther, A.; Wessling, M. Print your membrane: Rapid prototyping of complex 3D-PDMS membranes via a sacrificial resist. J. Memb. Sci. 2015, 478, 12–18. [Google Scholar] [CrossRef]
- Fritzmann, C.; Hausmann, M.; Wiese, M.; Wessling, M.; Melin, T. Microstructured spacers for submerged membrane filtration systems. J. Memb. Sci. 2013, 446, 189–200. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tan, W.S.; An, J.; Chua, C.K.; Tang, C.Y.; Fane, A.G.; Tzyy, H.C. The potential to enhance membrane module design with 3D printing technology. J. Memb. Sci. 2016, 499, 480–490. [Google Scholar] [CrossRef]
- Femmer, T.; Kuehne, A.J.C.; Wessling, M. Print your own membrane: Direct rapid prototyping of polydimethylsiloxane. Lab Chip 2014, 14, 2610–2613. [Google Scholar] [CrossRef] [PubMed]
- Breiter, S. Membranes for oxygenators and plasma filters. In Biomaterials for Artificial Organs; Lysaght, M., Webster, T.J., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–33. [Google Scholar]
- Pflaum, M.; Peredo, A.S.; Dipresa, D.; De, A.; Korossis, S. Membrane bioreactors for (bio-)artificial lung. In Current Trends and Future Developments on (Bio-)Membranes; Basile, A., Annesini, M.C., Piemonte, V., Charcosset, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 45–75. [Google Scholar]
- Kim, J. Recent Progress on Improving the Sustainability of Membrane Fabrication. J. Membr. Sci. Res. 2019. [Google Scholar] [CrossRef]
- Grace, H.P. Dispersion phenomena in high viscosity immiscible fluid systems and application of static mixers as dispersion devices in such systems. Chem. Eng. Commun. 1982, 14, 225–277. [Google Scholar] [CrossRef]
- Minale, M.; Moldenaers, P.; Mewis, J. Effect of shear history on the morphology of immiscible polymer blends. Macromolecules 1997, 30, 5470–5475. [Google Scholar] [CrossRef]
- Willemse, R.C.; Ramaker, E.J.J.; Van Dam, J.; De Boer, A.P. Morphology development in immiscible polymer blends: Initial blend morphology and phase dimensions. Polymer 1999, 40, 6651–6659. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.L.; Favis, B.D. The role of the blend interface type on morphology in cocontinuous polymer blends. Macromolecules 2005, 35, 2005–2016. [Google Scholar] [CrossRef]
- Minale, M. Models for the deformation of a single ellipsoidal drop: A review. Rheol. Acta 2010, 49, 789–806. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Polyakova, M.Y.; Kulichikhin, V.G. Phase behavior and rheology of miscible and immiscible blends of linear and hyperbranched siloxane macromolecules. Mater. Today Commun. 2020, 22, 100833. [Google Scholar] [CrossRef]
- Barnes, H.A. A review of the slip (wall depletion) of polymer solutions, emulsions and particle suspensions in viscometers: Its cause, character, and cure. J. Non-Newton. Fluid Mech. 1995, 56, 221–251. [Google Scholar] [CrossRef]
- Hatzikiriakos, S.G. Wall slip of molten polymers. Prog. Polym. Sci. 2012, 37, 624–643. [Google Scholar] [CrossRef]
- Kostyuk, A.; Ignatenko, V.; Smirnova, N.; Brantseva, T.; Ilyin, S.; Antonov, S. Rheology and adhesive properties of filled PIB-based pressure-sensitive adhesives. I. Rheology and shear resistance. J. Adhes. Sci. Technol. 2015, 29, 1831–1848. [Google Scholar] [CrossRef]
- Brantseva, T.; Antonov, S.; Kostyuk, A.; Ignatenko, V.; Smirnova, N.; Korolev, Y.; Tereshin, A.; Ilyin, S. Rheological and adhesive properties of PIB-based pressure-sensitive adhesives with montmorillonite-type nanofillers. Eur. Polym. J. 2016, 76, 228–244. [Google Scholar] [CrossRef]
- Utracki, L.A. On the viscosity-concentration dependence of immiscible polymer blends. J. Rheol. 1991, 35, 1615–1637. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Malkin, A.Y.; Kulichikhin, V.G.; Shaulov, A.Y.; Stegno, E.V.; Berlin, A.A.; Patlazhan, S.A. Rheological properties of polyethylene/metaboric acid thermoplastic blends. Rheol. Acta 2014, 53, 467–475. [Google Scholar] [CrossRef]
- Benderly, D.; Siegmann, A.; Narkis, M. Polymer encapsulation of glass filler in ternary PP/PA-6/glass blends. Polym. Compos. 1996, 17, 86–95. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Petrukhina, N.N.; Kostyuk, A.V.; Dzhabarov, E.G.; Filatova, M.P.; Antonov, S.V.; Maksimov, A.L. Hydrogenation of Indene–Coumarone Resin on Palladium Catalysts for Use in Polymer Adhesives. Russ. J. Appl. Chem. 2019, 92, 1143–1152. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kostyuk, A.V.; Ignatenko, V.Y.; Smirnova, N.M.; Alekseeva, O.A.; Petrukhina, N.N.; Antonov, S.V. The Effect of Tackifier on the Properties of Pressure-Sensitive Adhesives Based on Styrene–Butadiene–Styrene Rubber. Russ. J. Appl. Chem. 2018, 91, 1945–1956. [Google Scholar] [CrossRef]
- Fisher, I.; Siegmann, A.; Narkis, M. The effect of interface characteristics on the morphology, rheology and thermal behavior of three-component polymer alloys. Polym. Compos. 2002, 23, 34–48. [Google Scholar] [CrossRef]




| PIB | MPIB, kDa | Melting | Crystallization | σadh, kPa | σstr, MPa | E, GPa | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tm, °C | ΔHm, J/g | ΔHm/CPMP, J/g | Tcr, °C | ΔHcr, J/g | ΔHcr/CPMP, J/g | |||||
| - | - | 233.0 | 24.6 | 24.6 | 202.9 | 26.1 | 26.1 | 0 | 14.4 | 0.36 |
| B15 | 75 | 228.6 | 16.6 | 30.3 | 205.4 | 11.7 | 21.3 | 10 | 5.1 | 0.17 |
| B50 | 340 | 229.5 | 13.9 | 25.4 | 204.1 | 12.2 | 22.2 | 0.8 | 9.1 | 0.20 |
| B100 | 1100 | 229.6 | 16.4 | 29.8 | 203.8 | 11.7 | 21.3 | 0.3 | 8.2 | 0.14 |
| PIB | Pwater, kg/m2hbar | R240 nm, % | R38 nm, % |
|---|---|---|---|
| B15 | 31,000 | 93 | 8 |
| B50 | 1.9 | 99 | 39 |
| B100 | 76 | 58 | 3 |
| CPIB(B50), % | σstr, MPa | E, GPa | Pwater, kg/m2hbar | R240 nm, % | R38 nm, % |
|---|---|---|---|---|---|
| 0 | 14.4 | 0.36 | non-permeable | ||
| 30 | 13.2 | 0.27 | |||
| 35 | 11.7 | 0.22 | |||
| 40 | 9.5 | 0.21 | 0.05 | - | - |
| 45 | 9.1 | 0.20 | 1.9 | 99 | 39 |
| 50 | 7.9 | 0.13 | 1100 | 91 | 36 |
| 55 | 3.3 | 0.09 | 3790 | 87 | 29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyin, S.; Ignatenko, V.; Anokhina, T.; Bakhtin, D.; Kostyuk, A.; Dmitrieva, E.; Antonov, S.; Volkov, A. Formation of Microfiltration Membranes from PMP/PIB Blends: Effect of PIB Molecular Weight on Membrane Properties. Membranes 2020, 10, 9. https://doi.org/10.3390/membranes10010009
Ilyin S, Ignatenko V, Anokhina T, Bakhtin D, Kostyuk A, Dmitrieva E, Antonov S, Volkov A. Formation of Microfiltration Membranes from PMP/PIB Blends: Effect of PIB Molecular Weight on Membrane Properties. Membranes. 2020; 10(1):9. https://doi.org/10.3390/membranes10010009
Chicago/Turabian StyleIlyin, Sergey, Viktoria Ignatenko, Tatyana Anokhina, Danila Bakhtin, Anna Kostyuk, Evgenia Dmitrieva, Sergey Antonov, and Alexey Volkov. 2020. "Formation of Microfiltration Membranes from PMP/PIB Blends: Effect of PIB Molecular Weight on Membrane Properties" Membranes 10, no. 1: 9. https://doi.org/10.3390/membranes10010009