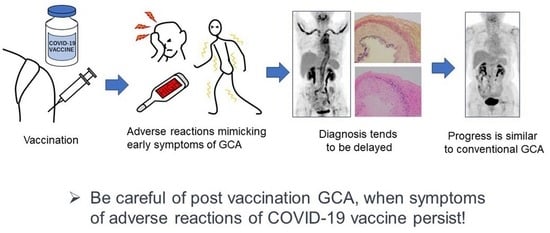
Giant Cell Arteritis after COVID-19 Vaccination with Long-Term Follow-Up: A Case Report and Review of the Literature
Abstract
:1. Introduction
2. Case Presentation
3. Discussion
- [clinical criteria]
- (1)
- morning stiffness in the shoulders/neck (+2)
- (2)
- sudden visual loss (+3)
- (3)
- jaw or tongue claudication (+2)
- (4)
- new temporal headache (+2)
- (5)
- scalp tenderness (+2)
- (6)
- abnormal examination of the temporal artery (+2)
- [test/imaging/biopsy criteria]
- (7)
- maximum ESR ≥50 mm/hour or maximum CRP ≥ 10 mg/L (+3)
- (8)
- positive temporal artery biopsy or halo sign on temporal artery ultrasound (+5)
- (9)
- bilateral axillary involvement (+2)
- (10)
- PET activity throughout the aorta (+2)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connolly, C.M.; Ruddy, J.A.; Boyarsky, B.J.; Barbur, I.; Werbel, W.A.; Geetha, D.; Garonzik-Wang, J.M.; Segev, D.L.; Christopher-Stine, L.; Paik, J.J. Disease Flare and Reactogenicity in Patients With Rheumatic and Musculoskeletal Diseases Following Two-Dose SARS-CoV-2 Messenger RNA Vaccination. Arthritis Rheumatol. 2022, 74, 28–32. [Google Scholar] [CrossRef]
- Mukhtyar, C.; Guillevin, L.; Cid, M.C.; Dasgupta, B.; de Groot, K.; Gross, W.; Hauser, T.; Hellmich, B.; Jayne, D.; Kallenberg, C.G.; et al. EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2009, 68, 318–323. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Vazquez-Rodriguez, T.R.; Lopez-Diaz, M.J.; Miranda-Filloy, J.A.; Gonzalez-Juanatey, C.; Martin, J.; Llorca, J. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009, 61, 1454–1461. [Google Scholar] [CrossRef]
- Samson, M.; Jacquin, A.; Audia, S.; Daubail, B.; Devilliers, H.; Petrella, T.; Martin, L.; Durier, J.; Besancenot, J.F.; Lorcerie, B.; et al. Stroke associated with giant cell arteritis: A population-based study. J. Neurol. Neurosurg. Psychiatry 2015, 86, 216–221. [Google Scholar] [CrossRef]
- Hunder, G.G.; Bloch, D.A.; Michel, B.A.; Stevens, M.B.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990, 33, 1122–1128. [Google Scholar] [CrossRef]
- Stamatis, P.; Turkiewicz, A.; Englund, M.; Jönsson, G.; Nilsson, J.; Turesson, C.; Mohammad, A.J. Infections Are Associated With Increased Risk of Giant Cell Arteritis: A Population-based Case-control Study from Southern Sweden. J. Rheumatol. 2021, 48, 251–257. [Google Scholar] [CrossRef]
- Salvarani, C.; Gabriel, S.E.; O’Fallon, W.M.; Hunder, G.G. The incidence of giant cell arteritis in Olmsted County, Minnesota: Apparent fluctuations in a cyclic pattern. Ann. Intern. Med. 1995, 123, 192–194. [Google Scholar] [CrossRef]
- Elling, P.; Olsson, A.T.; Elling, H. Synchronous variations of the incidence of temporal arteritis and polymyalgia rheumatica in different regions of Denmark; association with epidemics of Mycoplasma pneumoniae infection. J. Rheumatol. 1996, 23, 112–119. [Google Scholar]
- Teng, G.G.; Chatham, W.W. Vasculitis related to viral and other microbial agents. Best. Pract. Res. Clin. Rheumatol. 2015, 29, 226–243. [Google Scholar] [CrossRef]
- Quartuccio, L.; Treppo, E.; Dejaco, C. The pre-clinical phase of giant cell arteritis: New clues in the pathogenesis of giant cell arteritis supporting emerging targets. Rheumatology 2023, 62, 2032–2034. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Manzo, V.E.; Pedamallu, C.S.; Duke, F.; Cai, D.; Bienfang, D.C.; Padera, R.F.; Meyerson, M.; Docken, W.P. In search of a candidate pathogen for giant cell arteritis: Sequencing-based characterization of the giant cell arteritis microbiome. Arthritis Rheumatol. 2014, 66, 1939–1944. [Google Scholar] [CrossRef]
- Cadiou, S.; Perdriger, A.; Ardois, S.; Albert, J.D.; Berthoud, O.; Lescoat, A.; Guggenbuhl, P.; Robin, F. SARS-CoV-2, polymyalgia rheumatica and giant cell arteritis: COVID-19 vaccine shot as a trigger? Comment on: “Can SARS-CoV-2 trigger relapse of polymyalgia rheumatica?” by Manzo et al. Joint Bone Spine 2021;88:105150. Jt. Bone Spine 2022, 89, 105282. [Google Scholar] [CrossRef]
- Sauret, A.; Stievenart, J.; Smets, P.; Olagne, L.; Guelon, B.; Aumaitre, O.; Andre, M.; Trefond, L. Case of Giant Cell Arteritis After SARS-CoV-2 Vaccination: A Particular Phenotype? J. Rheumatol. 2022, 49, 120. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Edwards, R.; Omidvar, B. A Case of Giant Cell Arteritis With a Normal Erythrocyte Sedimentation Rate (ESR) Post ChAdOx1 nCoV-19 Vaccination. Cureus 2022, 14, e25388. [Google Scholar] [CrossRef] [PubMed]
- Lo Sardo, L.; Parisi, S.; Ditto, M.C.; De Giovanni, R.; Maletta, F.; Grimaldi, S.; Brussino, L.; Fusaro, E. New Onset of Giant Cell Arteritis following ChAdOx1-S (Vaxevria((R))) Vaccine Administration. Vaccines 2023, 11, 434. [Google Scholar] [CrossRef]
- Greb, C.S.; Aouhab, Z.; Sisbarro, D.; Panah, E. A Case of Giant Cell Arteritis Presenting After COVID-19 Vaccination: Is It Just a Coincidence? Cureus 2022, 14, e21608. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Krogias, C.; Tischoff, I.; Tannapfel, A.; Gold, R.; Susok, L. Bilateral giant cell arteritis with skin necrosis following SARS-CoV-2 vaccination. Br. J. Dermatol. 2022, 186, e83. [Google Scholar] [CrossRef]
- Mejren, A.; Sorensen, C.M.; Gormsen, L.C.; Tougaard, R.S.; Nielsen, B.D. Large-vessel giant cell arteritis after COVID-19 vaccine. Scand. J. Rheumatol. 2022, 51, 154–155. [Google Scholar] [CrossRef]
- Anzola, A.M.; Trives, L.; Martinez-Barrio, J.; Pinilla, B.; Alvaro-Gracia, J.M.; Molina-Collada, J. New-onset giant cell arteritis following COVID-19 mRNA (BioNTech/Pfizer) vaccine: A double-edged sword? Clin. Rheumatol. 2022, 41, 1623–1625. [Google Scholar] [CrossRef]
- Che, S.A.; Lee, K.Y.; Yoo, Y.J. Bilateral Ischemic Optic Neuropathy From Giant Cell Arteritis Following COVID-19 Vaccination. J. Neuroophthalmol. 2022, 43, e107–e108. [Google Scholar] [CrossRef]
- Gilio, M.; De Stefano, G. Large-vessel vasculitis following the Pfizer-BioNTech COVID-19 vaccine. Intern. Emerg. Med. 2022, 17, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, D.K.; Katayama, K.; Ohira, Y. Giant cell arteritis presenting with chronic cough and headache after BNT162b2 mRNA COVID-19 vaccination. QJM 2022, 115, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Iwayanagi, M.; Sakai, D.; Sugiura, Y.; Hiruta, N.; Matsuzawa, Y.; Kaneko, K. Development of giant cell arteritis after vaccination against SARS-CoV2: A case report and literature review. Medicine 2023, 102, e33948. [Google Scholar] [CrossRef]
- van Nieuwland, M.; Colin, E.M.; Boumans, D.; Vermeer, M.; Brouwer, E.; Alves, C. Diagnostic delay in patients with giant cell arteritis: Results of a fast-track clinic. Clin. Rheumatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J. Diseases of the arteries. Arch. Surg. 1890, 1, 323–333. [Google Scholar]
- Horton, B.T. An undescribed form of arteritis of thetemporal vessels. Proc. Staff. Meet. Mayo Clin. 1932, 7, 700–701. [Google Scholar]
- Klein, R.G.; Hunder, G.G.; Stanson, A.W.; Sheps, S.G. Large artery involvement in giant cell (temporal) arteritis. Ann. Intern. Med. 1975, 83, 806–812. [Google Scholar] [CrossRef]
- Lyne, S.A.; Ruediger, C.; Lester, S.; Chapman, P.T.; Shanahan, E.M.; Hill, C.L.; Stamp, L. Giant cell arteritis: A population-based retrospective cohort study exploring incidence and clinical presentation in Canterbury, Aotearoa New Zealand. Front. Med. 2022, 9, 1057917. [Google Scholar] [CrossRef]
- Ostberg, G. Temporal arteritis in a large necropsy series. Ann. Rheum. Dis. 1971, 30, 224–235. [Google Scholar] [CrossRef]
- Ostberg, G. Morphological changes in the large arteries in polymyalgia arteritica. Acta Med. Scand. Suppl. 1972, 533, 135–159. [Google Scholar] [CrossRef]
- Schmidt, W.A.; Seifert, A.; Gromnica-Ihle, E.; Krause, A.; Natusch, A. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology 2008, 47, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Ghinoi, A.; Pipitone, N.; Nicolini, A.; Boiardi, L.; Silingardi, M.; Germanò, G.; Salvarani, C. Large-vessel involvement in recent-onset giant cell arteritis: A case-control colour-Doppler sonography study. Rheumatology 2012, 51, 730–734. [Google Scholar] [CrossRef]
- Aschwanden, M.; Kesten, F.; Stern, M.; Thalhammer, C.; Walker, U.A.; Tyndall, A.; Jaeger, K.A.; Hess, C.; Daikeler, T. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2x11 arterial regions. Ann. Rheum. Dis. 2010, 69, 1356–1359. [Google Scholar] [CrossRef]
- Prieto-González, S.; Arguis, P.; García-Martínez, A.; Espígol-Frigolé, G.; Tavera-Bahillo, I.; Butjosa, M.; Sánchez, M.; Hernández-Rodríguez, J.; Grau, J.M.; Cid, M.C. Large vessel involvement in biopsy-proven giant cell arteritis: Prospective study in 40 newly diagnosed patients using CT angiography. Ann. Rheum. Dis. 2012, 71, 1170–1176. [Google Scholar] [CrossRef]
- Blockmans, D.; de Ceuninck, L.; Vanderschueren, S.; Knockaert, D.; Mortelmans, L.; Bobbaers, H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: A prospective study of 35 patients. Arthritis Rheum. 2006, 55, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Corbera-Bellalta, M.; Audia, S.; Planas-Rigol, E.; Martin, L.; Cid, M.C.; Bonnotte, B. Recent advances in our understanding of giant cell arteritis pathogenesis. Autoimmun. Rev. 2017, 16, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Dua, A.B.; Husainat, N.M.; Kalot, M.A.; Byram, K.; Springer, J.M.; James, K.E.; Chang Lin, Y.; Turgunbaev, M.; Villa-Forte, A.; Abril, A.; et al. Giant Cell Arteritis: A Systematic Review and Meta-Analysis of Test Accuracy and Benefits and Harms of Common Treatments. ACR Open Rheumatol. 2021, 3, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Ponte, C.; Grayson, P.C.; Robson, J.C.; Suppiah, R.; Gribbons, K.B.; Judge, A.; Craven, A.; Khalid, S.; Hutchings, A.; Watts, R.A.; et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022, 74, 1881–1889. [Google Scholar] [CrossRef]
- van Nieuwland, M.; van Bon, L.; Vermeer, M.; Brouwer, E.; Alves, C. External validation of the 2022 ACR/EULAR classification criteria in patients with suspected giant cell arteritis in a Dutch fast-track clinic. RMD Open 2023, 9, e003080. [Google Scholar] [CrossRef]
- Stone, J.H.; Tuckwell, K.; Dimonaco, S.; Klearman, M.; Aringer, M.; Blockmans, D.; Brouwer, E.; Cid, M.C.; Dasgupta, B.; Rech, J.; et al. Trial of Tocilizumab in Giant-Cell Arteritis. N. Engl. J. Med. 2017, 377, 317–328. [Google Scholar] [CrossRef]
- Maz, M.; Chung, S.A.; Abril, A.; Langford, C.A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis Care Res. 2021, 73, 1071–1087. [Google Scholar] [CrossRef]
- Hellmich, B.; Agueda, A.; Monti, S.; Buttgereit, F.; de Boysson, H.; Brouwer, E.; Cassie, R.; Cid, M.C.; Dasgupta, B.; Dejaco, C.; et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2020, 79, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Muratore, F.; Kermani, T.A.; Crowson, C.S.; Green, A.B.; Salvarani, C.; Matteson, E.L.; Warrington, K.J. Large-vessel giant cell arteritis: A cohort study. Rheumatology 2015, 54, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Liozon, E.; Parreau, S.; Filloux, M.; Dumonteil, S.; Gondran, G.; Bezanahary, H.; Ly, K.H.; Fauchais, A.L. Giant cell arteritis or polymyalgia rheumatica after influenza vaccination: A study of 12 patients and a literature review. Autoimmun. Rev. 2021, 20, 102732. [Google Scholar] [CrossRef] [PubMed]
- Agger, W.A.; Deviley, J.A.; Borgert, A.J.; Rasmussen, C.M. Increased Incidence of Giant Cell Arteritis After Introduction of a Live Varicella Zoster Virus Vaccine. Open Forum. Infect. Dis. 2021, 8, ofaa647. [Google Scholar] [CrossRef]
- Rijkers, G.T.; Weterings, N.; Obregon-Henao, A.; Lepolder, M.; Dutt, T.S.; van Overveld, F.J.; Henao-Tamayo, M. Antigen Presentation of mRNA-Based and Virus-Vectored SARS-CoV-2 Vaccines. Vaccines 2021, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Robinette, M.L.; Rao, D.A.; Monach, P.A. The Immunopathology of Giant Cell Arteritis Across Disease Spectra. Front. Immunol. 2021, 12, 623716. [Google Scholar] [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Moreno-Torres, V.; Soriano, V.; Calderon-Parra, J.; Martinez-Urbistondo, M.; Trevino, A.; de San Vicente, Z.; de Mendoza, C.; Ruiz-Irastorza, G. Increased incidence of giant cell arteritis and associated stroke during the COVID-19 pandemic in Spain: A nation-wide population study. Autoimmun. Rev. 2023, 22, 103341. [Google Scholar] [CrossRef]
- Tomelleri, A.; Sartorelli, S.; Baldissera, E.M.; Dagna, L.; Campochiaro, C. Response to: ‘Correspondence on ‘Impact of COVID-19 pandemic on patients with large-vessels vasculitis in Italy: A monocentric survey’ by Comarmond et al. Ann. Rheum. Dis. 2023, 82, e31. [Google Scholar] [CrossRef]
- Comarmond, C.; Leclercq, M.; Leroux, G.; Marques, C.; Le Joncour, A.; Domont, F.; Hatte, C.; Toquet-Bouedec, S.; Guillaume-Jugnot, P.; Desbois, A.C.; et al. Correspondence on ‘Impact of COVID-19 pandemic on patients with large-vessels vasculitis in Italy: A monocentric survey’. Ann. Rheum. Dis. 2023, 82, e30. [Google Scholar] [CrossRef] [PubMed]
- Lecler, A.; Villeneuve, D.; Vignal, C.; Sene, T. Increased rather than decreased incidence of giant-cell arteritis during the COVID-19 pandemic. Ann. Rheum. Dis. 2021, 80, e89. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.; Tomelleri, A.; Buttgereit, F.; Matteson, E.L.; Dejaco, C. Looking ahead: Giant-cell arteritis in 10 years time. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221096366. [Google Scholar] [CrossRef] [PubMed]



| Test Name | At the Time of Arrival | 27 Days after Treatment | Reference Range |
|---|---|---|---|
| White blood cells (μL) | 8300 | 8200 | 3300–8600 |
| Red blood cells (104/μL) | 254 | 336 | 386–492 |
| Hemoglobin (g/dL) | 7.6 | 10.4 | 11.6–14.8 |
| Hematocrit (%) | 22.9 | 31.8 | 35.1–44.4 |
| Platelets (104/μL) | 58.6 | 17.1 | 15.8–34.8 |
| ESR 1 h (mm) | >140 | 5 | 3–5 |
| D-dimer (μg/mL) | 1.7 | 1.4 | 0.0–1.0 |
| C-reactive protein (mg/L) | 211 | 0.1 | 0.0–1.4 |
| Aspartate aminotransferase (U/L) | 30 | 16 | 13–30 |
| Alanine transaminase (U/L) | 40 | 23 | 7–23 |
| Lactate dehydrogenase (U/L) | 137 | 200 | 124–222 |
| Alkaline phosphatase (U/L) | 106 | 76 | 106–322 |
| Gamma glutamyl transpeptidase (U/L) | 63 | 26 | 9–32 |
| Blood urea nitrogen (mg/dL) | 11 | 18 | 8–20 |
| Creatinine (mg/dL) | 0.7 | 0.76 | 0.46–0.79 |
| Total bilirubin (mg/dL) | 0.6 | 0.9 | 0.4–1.5 |
| Procalcitonin (ng/mL) | 0.12 | - | <0.05 |
| 50% hemolytic complement activity (CH50) (U/mL) | 63 | - | 30–46 |
| Complement component 3 (C3) (mg/dL) | 156 | - | 73–138 |
| Complement component 4 (C4) (mg/dL) | 37 | - | 11–31 |
| IgA (mg/dL) | 275 | - | 93–393 |
| IgG (mg/dL) | 1697 | - | 861–1747 |
| IgM (mg/dL) | 55 | - | 50–269 |
| IgG4 (mg/dL) | 91 | 11–121 | |
| PR3-ANCA (U/mL) | <1.0 | - | <1.0 |
| MPO-ANCA (U/mL) | <1.0 | - | <1.0 |
| Antinuclear Antibodies | 1:80 | - | <40 |
| Ferritin (ng/mL) | 507 | 370 * | <55 |
| Soluble interleukin-2 receptor (U/mL) | 712 | -- | 157–474 |
| Blood culture | Negative | - | Negative |
| Polymerase chain reaction (PCR) for SARS-CoV-2 | Negative | - | Negative |
| Reference | Sex, Age | Past Medical History | Symptoms | Type of Vaccine | Number of Vx | Time from Vx to Onset | Time from Onset to Dx | Mode of Dx | Result of Biopsy | LV-GCA or C-GCA | Arteritis Location | CRP (mg/L) | ESR (mm/h) | Treatment | Time to Improvement | Treatment Duration | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cadiou et al. [12] | F, 70 | PMR | Fatigue | Viral vector | 2 | 10 days | 3 weeks | PET | Not described | LV-GCA | A panaortic and supra-aortic vasculitis (PET) | 104 | - | PSL 40 mg | 1 month | More than 11 weeks | Discharge |
| Sauret et al. [13] | M, 70 | Not described | Headache, hyperesthesia of the scalp | Viral vector | 1 | Few days | Not described | Biopsy | GCA | C-GCA | Temporal artery (PET, No large vessel vasculitis) | 14 | - | PSL 0.5 mg/kg | Following day | Not described | Discharge |
| Xia et al. [14] | M, 68 | Chronic obstructive pulmonary disease | Right-sided temporal headache, blurred vision, bilateral jaw claudication | Viral vector | 2 | 3–5 days | 3 weeks | Biopsy | GCA | C-GCA | Temporal artery (Angiogram normal) | 29 | 4 | 1 g mPSL pulse, PSL 65 mg, TCZ 162 mg | PSL 65 mg only, blurred vision on opposite side after 3 months, improved after 3 months with TCZ | More than 6 months | Discharge |
| Sardo et al. [15] | M, 78 | Melanoma, HTN Hepatitis B infection, | Headache, fatigue, jaw claudication, scotomas, pharyngalgia, dry cough | Viral vector | 2 | 1 day | 1 month | Biopsy | GCA | LV-GCA | Ascending aorta, aortic arch, descending aorta, iliac axes bilaterally and subclavian bilaterally (PET) | 84 | - | PSL 1 mg/kg TCZ | A few weeks | More than 8 months | Discharge |
| Greb et al. [16] | M, 79 | HTN, hyperlipidemia, atrial fibrillation, hypothyroidism, prostate cancer, rectal cancer | Headache, transient blurry vision, fever, fatigue, myalgias | mRNA | 2 | 2 days | 1 month | Biopsy | GCA | C-GCA | Temporal artery | 272 | 97 | PSL 60 mg | 3 weeks | More than 6 weeks | Discharge |
| Cadiou et al. [12] | F, 74 | Advanced ovarian cancer | Headache, jaw claudication | mRNA | 1 | 7 days | 5 weeks | Biopsy | GCA | C-GCA | Temporal artery | 190 | - | PSL 60 mg | 1 week | Not described | Discharge |
| Gambichler et al. [17] | M, 82 | Not described | Headaches, jaw claudication, weight loss, bilateral temporoparietal skin necrosis, vision loss | mRNA | 2 | 10 days | 4 months | Biopsy | GCA | C-GCA | Temporal artery | 63 | - | Not described | Not described | Not described | Discharge (Complete vision loss before treatment) |
| Mejren et al. [18] | F, 62 | Not described | Fatigue, weight loss, night sweat, nausea | mRNA | 1, 2 | 1–2 days | 7–8 weeks | PET | Not described | LV-GCA | The vertebral, common carotid, maxillary, axillary, subclavian, internal mammary, common iliac arteries, throughout the aorta (PET) | 98 | - | PSL 40 mg | 2 weeks | Not described | Discharge |
| Anzola et al. [19] | F, 83 | Dyslipidemia, HTN | Disruptive cervical pain, headache, scalp tenderness | mRNA | 1 | 1 day | 3 weeks | PET | Normal | LV-GCA | Bilateral vertebral artery (PET) | 14 | 71 | Pulse steroids, methotrexate, medium-dose steroids | 3 months | More than 6 months | Discharge (Remission weekly methotrexate and low-dose steroids) |
| Che et al. [20] | F, 87 | HTN | Visual loss, scalp tenderness, fever | mRNA | 1 | 1 day | 2 weeks | Biopsy | GCA | C-GCA | Temporal artery | 8 | 120 | Pulse steroids, PSL 60 mg | 4 months | More than 6 months | Discharge (Right eye complete vision loss, left eye blurred vision improved) |
| Gilio et al. [21] | F, 63 | HTN | Fatigue, myalgias, low grade fevers, anorexia, headache, arthralgia, stiffness of upper arms, shoulders and neck | mRNA | 1 | 1 day | 1 month | PET | Not described | LV-GCA | Aortic arch, thoracic and abdominal aorta, carotid, subclavian arteries (PET) | 74 | 104 | PSL 50 mg | 4 weeks | Not described (tapering ongoing) | Discharge (Tapering ongoing) |
| Ishizuka et al. [22] | M, 74 | HTN | Cough, left temporal headache | mRNA | 3 | 1 day | 2 months | PET | Not described | LV-GCA | Thoracic aorta, subclavian, axillary, brachial and temporal arteries (PET) | 63 | 79 | PSL 30 mg | Not described | Not described | Not described (Symptoms improved) |
| Wakabayashi et al. [23] | M, 77 | Type 2 diabetes mellitus, Basedow’s disease, prostate cancer | Fatigue, headache, nodular swelling and tenderness of the bilateral temporal arteries | mRNA | 3 | 1 day | 3 months | Echography | Normal | C-GCA | Temporal artery (Echography) | 134 | 62 | 1 g mPSL pulse PSL 1 mg/kg TCZ 162 mg | 16 days | More than 45 days | Discharge (PSL 30 mg) |
| Yoshimoto et al. (this case) | F, 69 | None | Headache, fever, abdominal pain, body weight loss | mRNA | 1 | 2 days | 38 days (5 weeks) | PET | GCA | LV-GCA | Ascending aorta, aortic arch, descending aorta, bilateral subclavian and iliac artery (PET) | 211 | 140 | 1g mPSL pulse PSL 50 mg TCZ 162 mg | 2 weeks | 2 years | Discharge (Remission with PSL 3 mg & TCZ) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimoto, K.; Kaneda, S.; Asada, M.; Taguchi, H.; Kawashima, H.; Yoneima, R.; Matsuoka, H.; Tsushima, E.; Ono, S.; Matsubara, M.; et al. Giant Cell Arteritis after COVID-19 Vaccination with Long-Term Follow-Up: A Case Report and Review of the Literature. Medicina 2023, 59, 2127. https://doi.org/10.3390/medicina59122127
Yoshimoto K, Kaneda S, Asada M, Taguchi H, Kawashima H, Yoneima R, Matsuoka H, Tsushima E, Ono S, Matsubara M, et al. Giant Cell Arteritis after COVID-19 Vaccination with Long-Term Follow-Up: A Case Report and Review of the Literature. Medicina. 2023; 59(12):2127. https://doi.org/10.3390/medicina59122127
Chicago/Turabian StyleYoshimoto, Kiyomi, Saori Kaneda, Moe Asada, Hiroyuki Taguchi, Hiromasa Kawashima, Ryo Yoneima, Hidetoshi Matsuoka, Emiko Tsushima, Shiro Ono, Masaki Matsubara, and et al. 2023. "Giant Cell Arteritis after COVID-19 Vaccination with Long-Term Follow-Up: A Case Report and Review of the Literature" Medicina 59, no. 12: 2127. https://doi.org/10.3390/medicina59122127