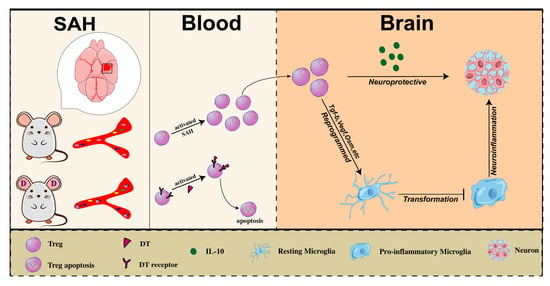
Regulatory T Cells Secrete IL10 to Suppress Neuroinflammation in Early Stage after Subarachnoid Hemorrhage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Animals
2.2. Reagents and Instruments
2.3. SAH Model
2.4. SAH Severity Assessment
2.5. Gracia’s Score Test
2.6. Brain Water Content Assessment
2.7. TUNEL Staining
2.8. Flow Cytometry
2.9. Behavioral Tests
2.10. Bulk RNA Sequencing and Analysis
2.11. Real Time Fluorescence Quantitative PCR (qPCR)
2.12. Hematoxylin-Eosin (HE) Staining
2.13. Statistics
3. Results
3.1. Time Course of Tregs Infiltrating into Brain of Mice after SAH
3.2. Transcriptome Change of Brain-Infiltrated Tregs after SAH
3.3. The Beneficial Effect of Tregs on Neurological Function in Mice after SAH
3.4. Exogenous Tregs Infusion Alleviated Brain Edema and Reduced Neuron Apoptosis in Mice after SAH
3.5. Tregs Exerted a Neuroprotective Effect by Suppressing Neuroimmune Inflammation at Early Stage of SAH
3.6. Brain-Infiltrated Tregs Exert Anti-Inflammatory Effect by Secreting IL-10 after SAH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawton, M.T.; Vates, G.E. Subarachnoid Hemorrhage. N. Engl. J. Med. 2017, 377, 257–266. [Google Scholar] [CrossRef]
- Andersen, C.R.; Presseau, J.; Saigle, V.; Etminan, N.; Vergouwen, M.D.I.; English, S.W. Core outcomes for subarachnoid haemorrhage. Lancet Neurol. 2019, 18, 1075–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Leak, R.; Thomson, A.W.; Yu, F.; Xia, Y.; Wechsler, L.R.; Chen, J. Promises and limitations of immune cell-based therapies in neurological disorders. Nat. Rev. Neurol. 2018, 14, 559–568. [Google Scholar] [CrossRef]
- Liesz, A.; Suri-Payer, E.; Veltkamp, C.; Doerr, H.; Sommer, C.; Rivest, S.; Giese, T.; Veltkamp, R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 2009, 15, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gan, Y.; Sun, B.-L.; Zhang, F.; Lu, B.; Gao, Y.; Liang, W.; Thomson, A.W.; Chen, J.; Hu, X. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann. Neurol. 2013, 74, 458–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Mao, L.; Liu, X.; Gan, Y.; Zheng, J.; Thomson, A.W.; Gao, Y.; Chen, J.; Hu, X. Essential role of program death 1-ligand 1 in regulatory T-cell-afforded protection against blood-brain barrier damage after stroke. Stroke 2014, 45, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Mao, L.; Zhang, L.; Zhang, L.; Yang, M.; Zhang, Z.; Li, D.; Fan, C.; Sun, B. Adoptive Regulatory T-cell Therapy Attenuates Subarachnoid Hemor-rhage-induced Cerebral Inflammation by Suppressing TLR4/NF-B Signaling Pathway. Curr. Neurovasc. Res. 2016, 13, 121–126. [Google Scholar] [CrossRef]
- Kim, J.M.; Rasmussen, J.P.; Rudensky, A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007, 8, 191–197. [Google Scholar] [CrossRef]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Dombrowski, Y.; O’Hagan, T.; Dittmer, M.; Penalva, R.; Mayoral, S.R.; Bankhead, P.; Fleville, S.; Eleftheriadis, G.; Zhao, C.; Naughton, M.; et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 2017, 20, 674–680. [Google Scholar] [CrossRef] [Green Version]
- Berg, D.J.; Davidson, N.; Kühn, R.; Müller, W.; Menon, S.; Holland, G.; Thompson-Snipes, L.; Leach, M.W.; Rennick, D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J. Clin. Investig. 1996, 98, 1010–1020. [Google Scholar] [CrossRef]
- Suzuki, H.; Hasegawa, Y.; Kanamaru, K.; Zhang, J.H. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke 2010, 41, 1783–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugawara, T.; Ayer, R.; Jadhav, V.; Zhang, J.H. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J. Neurosci. Methods 2008, 167, 327–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, J.H.; Wagner, S.; Liu, K.F.; Hu, X.J. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995, 26, 627–634; discussion 635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Q.; Zhang, Q.; Lu, Y.; Liu, J.; Li, W.; Lv, S.; Zhou, M.; Zhang, X.; Hang, C. Resveratrol Attenuates Early Brain Injury after Experimental Subarachnoid Hemorrhage via Inhibition of NLRP3 Inflammasome Activation. Front. Neurosci. 2017, 11, 611. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Pu, H.; Leak, R.K.; Shi, Y.; Mu, H.; Hu, X.; Lu, Z.; Foley, L.M.; Hitchens, T.K.; Dixon, C.E.; et al. Tissue plasminogen activator promotes white matter integrity and functional recovery in a murine model of traumatic brain injury. Proc. Natl. Acad. Sci. USA 2018, 115, E9230–E9238. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhu, W.; Xu, F.; Dai, X.; Shi, L.; Cai, W.; Mu, H.; Hitchens, T.K.; Foley, L.M.; Liu, X.; et al. The interleukin-4/PPARγ signaling axis promotes oligodendrocyte differentiation and remyelination after brain injury. PLoS Biol. 2019, 17, e3000330. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Walter, W.; Sánchez-Cabo, F.; Ricote, M. GOplot: An R package for visually combining expression data with functional analysis. Bioinformatics 2015, 31, 2912–2914. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.M.; Dejam, A.; Grimes, G.; Gladwin, M.T.; Oldfield, E.H. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. JAMA 2005, 293, 1477–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macdonald, R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2014, 10, 44–58. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke 2012, 43, 1463–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehba, F.A.; Hou, J.; Pluta, R.M.; Zhang, J.H. The importance of early brain injury after subarachnoid hemorrhage. Prog. Neurobiol. 2012, 97, 14–37. [Google Scholar] [CrossRef] [Green Version]
- Bosche, B.; Graf, R.; Ernestus, R.I.; Dohmen, C.; Reithmeier, T.; Brinker, G.; Strong, A.J.; Dreier, J.P.; Woitzik, J. Recurrent spreading depolarizations after subarachnoid hemorrhage decreases oxygen availability in human cerebral cortex. Ann. Neurol. 2010, 67, 607–617. [Google Scholar] [CrossRef]
- Shi, L.; Al-Baadani, A.; Zhou, K.; Shao, A.; Xu, S.; Chen, S.; Zhang, J. PCMT1 Ameliorates Neuronal Apoptosis by Inhibiting the Activation of MST1 after Subarachnoid Hemorrhage in Rats. Transl. Stroke Res. 2017, 8, 474–483. [Google Scholar] [CrossRef]
- Chen, S.; Ma, Q.; Krafft, P.R.; Hu, Q.; Rolland, I.I.W.; Sherchan, P.; Zhang, J.; Tang, J.; Zhang, J.H. P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiol. Dis. 2013, 58, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, K.; Zuo, Y.C.; Sherchan, P.; Wang, J.K.; Yan, X.X.; Liu, F. Hydrogen Inhalation Attenuates Oxidative Stress Related Endothelial Cells Injury After Subarachnoid Hemorrhage in Rats. Front. Neurosci. 2019, 13, 1441. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Feng, H.; Sherchan, P.; Klebe, D.; Zhao, G.; Sun, X.; Zhang, J.; Tang, J.; Zhang, J.H. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog. Neurobiol. 2014, 115, 64–91. [Google Scholar] [CrossRef] [Green Version]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Sakowitz, O.W.; Wolfrum, S.; Sarrafzadeh, A.S.; Stover, J.F.; Dreier, J.P.; Dendorfer, A.; Benndorf, G.; Lanksch, W.R.; Unterberg, A.W. Relation of cerebral energy metabolism and extracellular nitrite and nitrate concentrations in patients after aneurysmal subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2001, 21, 1067–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, J.M.; Avia, M.; Martín, V.; Sevilla, N. IL-10: A Multifunctional Cytokine in Viral Infections. J. Immunol. Res. 2017, 2017, 6104054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubtsov, Y.P.; Rasmussen, J.P.; Chi, E.Y.; Fontenot, J.; Castelli, L.; Ye, X.; Treuting, P.; Siewe, L.; Roers, A.; Henderson, W.R.; et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 2008, 28, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, W.D.; Greenhalgh, A.D.; Takwale, A.; David, S.; Vadigepalli, R. Novel Influences of IL-10 on CNS Inflammation Revealed by Integrated Analyses of Cytokine Networks and Microglial Morphology. Front. Cell. Neurosci. 2017, 11, 233. [Google Scholar] [CrossRef] [Green Version]
- Strle, K.; Zhou, J.H.; Broussard, S.R.; Venters, H.D.; Johnson, R.W.; Freund, G.G.; Dantzer, R.; Kelley, K.W. IL-10 promotes survival of microglia without activating Akt. J. Neuroimmunol. 2002, 122, 9–19. [Google Scholar] [CrossRef]
- Laffer, B.; Bauer, D.; Wasmuth, S.; Busch, M.; Jalilvand, T.V.; Thanos, S.; zu Horste, G.M.; Loser, K.; Langmann, T.; Heiligenhaus, A.; et al. Loss of IL-10 Promotes Differentiation of Microglia to a M1 Phenotype. Front. Cell. Neurosci. 2019, 13, 430. [Google Scholar] [CrossRef]
- Shi, L.; Sun, Z.; Su, W.; Xu, F.; Xie, D.; Zhang, Q.; Dai, X.; Iyer, K.; Hitchens, T.K.; Foley, L.M.; et al. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity 2021, 54, 1527–1542.e8. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; Cao, B.B.; Qiu, Y.H.; Peng, Y.P. Treg Cells Attenuate Neuroinflammation and Protect Neurons in a Mouse Model of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2020, 15, 224–237. [Google Scholar] [CrossRef]
- Beckmann, L.; Obst, S.; Labusek, N.; Abberger, H.; Köster, C.; Klein-Hitpass, L.; Schumann, S.; Kleinschnitz, C.; Hermann, D.M.; Felderhoff-Müser, U.; et al. Regulatory T Cells Contribute to Sexual Dimorphism in Neonatal Hypoxic-Ischemic Brain Injury. Stroke 2022, 53, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.A.; Peng, J.; Peckham, H.; Butler, G.; Pineda-Torra, I.; Ciurtin, C.; Jury, E.C. Investigating sex differences in T regulatory cells from cisgender and transgender healthy individuals and patients with autoimmune inflammatory disease: A cross-sectional study. Lancet Rheumatol. 2022, 4, e710–e724. [Google Scholar] [CrossRef] [PubMed]





| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Il1a | AAGACAAGCCTGTGTTGCTGAAGG | TCCCAGAAGAAAATGAGGTCGGTC |
| Il6 | TCCTACCCCAACTTCCAATGCTC | TTGGATGGTCTTGGTCCTTAGCC |
| Il10 | CCAAGCCTTATCGGAAATGA | TTTTCACAGGGGAGAAATCG |
| Ifng | ATGAACGCTACACACTGCATC | CCATCCTTTTGCCAGTTCCTC |
| Tnf | AGAAGTTCCCAAATGGCCTC | CCACTTGGTGGTTTGCTACG |
| Tgfb1 | TGCGCTTGCAGAGATTAAAA | CGTCAAAAGACAGCCACTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Yang, F.; Li, H.; Xu, P.; Wang, Z.; Shao, F.; Shao, A.; Zhang, J. Regulatory T Cells Secrete IL10 to Suppress Neuroinflammation in Early Stage after Subarachnoid Hemorrhage. Medicina 2023, 59, 1317. https://doi.org/10.3390/medicina59071317
Zhou J, Yang F, Li H, Xu P, Wang Z, Shao F, Shao A, Zhang J. Regulatory T Cells Secrete IL10 to Suppress Neuroinflammation in Early Stage after Subarachnoid Hemorrhage. Medicina. 2023; 59(7):1317. https://doi.org/10.3390/medicina59071317
Chicago/Turabian StyleZhou, Jingyi, Fan Yang, Huaming Li, Penglei Xu, Zefeng Wang, Fangjie Shao, Anwen Shao, and Jianmin Zhang. 2023. "Regulatory T Cells Secrete IL10 to Suppress Neuroinflammation in Early Stage after Subarachnoid Hemorrhage" Medicina 59, no. 7: 1317. https://doi.org/10.3390/medicina59071317