Development of Efficient One-Pot Methods for the Synthesis of Luminescent Dyes and Sol–Gel Hybrid Materials
Abstract
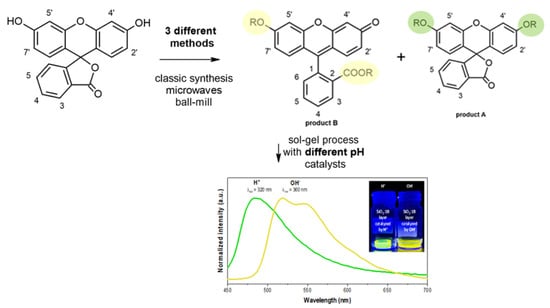
:1. Introduction
2. Materials and Methods
2.1. Synthesis Procedures
2.1.1. General Synthesis Procedure
2.1.2. General Procedure of Microwave-Assisted Fluorescein Alkylation
2.1.3. General Procedure of Solventless Ball-Milling Fluorescein Alkylation
2.1.4. General Procedure of the Sol–Gel Synthesis
3. Results and Discussion
3.1. Synthesis Optimization
3.2. New Route—Ball Milling
3.3. Spectroscopic Properties
3.4. Sol–Gel Hybrid Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baeyer, A. Ueber eine neue Klasse von Farbstoffen. Ber. Dtsch. Chem. Ges. 1871, 4, 555–558. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Zhan, X.-Q.; Bian, Q.-N.; Zhang, X.-J. Advances in modifying fluorescein and rhodamine fluorophores as fluorescent chemosensors. Chem. Commun. 2013, 49, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Zhang, J.; Liu, L. Fluorescence Properties of Twenty Fluorescein Derivatives: Lifetime, Quantum Yield, Absorption and Emission Spectra. J. Fluoresc. 2014, 24, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Mchedlov-Petrossyan, N.O.; Cheipesh, T.A.; Shekhovtsov, S.V.; Redko, A.N.; Rybachenko, V.I.; Omelchenko, I.V.; Shishkin, O.V. Ionization and tautomerism of methyl fluorescein and related dyes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Shamansky, L.M.; Yang, M.; Olteanu, M.; Chronister, E.L. A spectroscopic study of the pH sensor response of fluorescein doped silica and aluminosilica sol-gel glasses. Mater. Lett. 1996, 26, 113–120. [Google Scholar] [CrossRef]
- Yguerabide, J.; Talavera, E.; Alvarez, J.M.; Quintero, B. Steady-state fluorescence method for evaluating excited state proton reactions: Application to fluorescein. Photochem. Photobiol. 1994, 60, 435–441. [Google Scholar] [CrossRef]
- Gerasimova, M.A.; Tomilin, F.N.; Malyar, E.Y.; Varganov, S.A.; Fedorov, D.G.; Ovchinnikov, S.G.; Slyusareva, E.A. Fluorescence and photoinduced proton transfer in the protolytic forms of fluorescein: Experimental and computational study. Dye. Pigment. 2020, 173, 107851. [Google Scholar] [CrossRef]
- Ramart-Lucas, P. Structure et absorption des colorants. Formes isomères de la fluorescéine. Comptes Rendus 1937, 205, 1409. [Google Scholar]
- Bogdanova, L.N.; Mchedlov-Petrossyan, N.O.; Vodolazkaya, N.A.; Lebed, A.V. The influence of β-cyclodextrin on acid–base and tautomeric equilibrium of fluorescein dyes in aqueous solution. Carbohydr. Res. 2010, 345, 1882–1890. [Google Scholar] [CrossRef]
- Batistela, V.R.; da Costa Cedran, J.; Moisés de Oliveira, H.P.; Scarminio, I.S.; Ueno, L.T.; Eduardo da Hora Machado, A.; Hioka, N. Protolytic fluorescein species evaluated using chemometry and DFT studies. Dye. Pigment. 2010, 86, 15–24. [Google Scholar] [CrossRef]
- Mtschedlow-Petrossjan, N.O.; Kordowa, E.A.; Schapowalow, S.A.; Rappoport, I.A.; Egorowa, S.I. Zur Frage der Struktur der einwertigen Fiuoresceinfarbstoffanionen in Lösungen. Z. Chem. 2010, 30, 442–443. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Salinas Mayorga, R.; Surov, Y. Ionization and tautomerism of xanthene dyes in water-dimethyl sulfoxide mixtures. Zhurnal Obs. Khimii 1991, 61, 225–233. [Google Scholar]
- Ding, Z.; Wang, C.; Fan, M.; Zhang, M.; Zhou, Y.; Cui, X.; Zhang, D.; Wang, T. Far-red imaging of β-galactosidase through a phospha-fluorescein. Chem. Commun. 2020, 56, 13579–13582. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.H.; Choi, J.H.; Been, S.Y.; Kim, N.; Choi, J.M.; Kim, W.; Kim, D.; Jung, J.J.; Song, J.E.; Khang, G. Development of fluorescein isothiocyanate conjugated gellan gum for application of bioimaging for biomedical application. Int. J. Biol. Macromol. 2020, 164, 2804–2812. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Park, W.H. Fluorescent property of glycol chitosan-fluorescein isothiocyanate conjugate for bio-imaging material. Int. J. Biol. Macromol. 2019, 135, 1217–1221. [Google Scholar] [CrossRef]
- Koktysh, D.S. Ratiometric pH sensor using luminescent CuInS2/ZnS quantum dots and fluorescein. Mater. Res. Bull. 2020, 123, 110686. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Shimada, K.; Matsui, K. Spectroscopic study of fluorescein immobilized on anodic porous alumina in aqueous solutions of different pH. Dye. Pigment. 2020, 173, 107944. [Google Scholar] [CrossRef]
- Imhof, A.; Megens, M.; Engelberts, J.J.; De Lang, D.T.N.; Sprik, R.; Vos, W.L. Spectroscopy of fluorescein (FITC) dyed colloidal silica spheres. J. Phys. Chem. B 1999, 103, 1408–1415. [Google Scholar] [CrossRef] [Green Version]
- Boonsin, R.; Chadeyron, G.; Roblin, J.P.; Boyer, D.; Mahiou, R. Silica encapsulated fluorescein as a hybrid dye for blue-LED based lighting devices. J. Mater. Chem. C 2016, 4, 6562–6569. [Google Scholar] [CrossRef]
- Xie, C.; Yin, D.; Li, J.; Zhang, L.; Liu, B.; Wu, M. Preparation of a Novel Amino Functionalized Fluorescein-doped Silica Nanoparticle for pH Probe. Nano Biomed. Eng. 2009, 1, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Benkö, G.; Skårman, B.; Wallenberg, R.; Hagfeldt, A.; Sundström, V.; Yartsev, A.P. Particle size and crystallinity dependent electron injection in fluorescein 27-sensitized TiO2 films. J. Phys. Chem. B 2003, 107, 1370–1375. [Google Scholar] [CrossRef]
- Yang, Y.; Wallace, P.A.; Campbell, M.; Smith, S. Alteration in the response of fluorescein immobilized in sol-gel thin films as an optical fiber sensing mechanism for pH. In Proceedings of the SPIE Photonics China ’96, Beijing, China, 4 November 1996; Volume 53, pp. 237–242. [Google Scholar]
- Huang, L.; Wei, D.; Wu, Z.; Hou, Y.; Xie, Y.; Liu, Z.; Wang, C.; Che, D.; Lei, Y.; He, H. 5-hydroxymethylfurfural- and fluorescein-fused fluorescence probe of mast cells (RBL-2H3): Synthesis, photophysical properties, and bioimaging. Chem. Biol. Drug Des. 2018, 92, 1963–1971. [Google Scholar] [CrossRef]
- Rao, H.; Wang, P.; Li, C.J. Visible-light-triggered direct benzoyloxylation of electron-rich arenes at room temperature without chelation assistance. Eur. J. Org. Chem. 2012, 6503–6507. [Google Scholar] [CrossRef]
- Bouchard, A.; Deng, Y.; Dombrowski, E.J.; Gaudiana, R.A.; Haque, S.; Hasan, F.B.; Marshall, J.L.; Telfer, S.J.; Vetterling, W.T.; Viola, M.S.; et al. Thermal Transfer Recording System Having an Amorphous Dye Phase. Patent WO2001056805, 9 August 2001. [Google Scholar]
- More, K.N.; Lim, T.-H.; Kim, S.-Y.; Kang, J.; Inn, K.-S.; Chang, D.-J. Characteristics of new bioreductive fluorescent probes based on the xanthene fluorophore: Detection of nitroreductase and imaging of hypoxic cells. Dye. Pigment. 2018, 151, 245–253. [Google Scholar] [CrossRef]
- Xiong, H.; Li, R.-R.; Liu, S.-Y.; Wu, F.-X.; Yang, W.-C.; Yang, G.-F. Discovery of Specific Nonpeptide Probe for Chymotrypsin via Molecular Docking-Based Virtual Screening and the Application. ACS Appl. Bio Mater. 2018, 1, 310–317. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Han, B.; Zhu, B.; Zhang, X. A highly selective fluorescent chemodosimeter for imaging hydrogen sulfide in living cells. Dye. Pigment. 2014, 110, 214–218. [Google Scholar] [CrossRef]
- Fu, Q.; Li, H.; Duan, D.; Wang, C.; Shen, S.; Ma, H.; Liu, Z. External-Radiation-Induced Local Hydroxylation Enables Remote Release of Functional Molecules in Tumors. Angew. Chem. Int. Ed. 2020, 59, 21546–21552. [Google Scholar] [CrossRef]
- Feng, S.; Gong, S.; Feng, G. Aggregation-induced emission and solid fluorescence of fluorescein derivatives. Chem. Commun. 2020, 56, 2511–2513. [Google Scholar] [CrossRef] [PubMed]
- Skoreński, M.; Milewska, A.; Pyrć, K.; Sieńczyk, M.; Oleksyszyn, J. Phosphonate inhibitors of West Nile virus NS2B/NS3 protease. J. Enzym. Inhib. Med. Chem. 2019, 34, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, O.; Hepp, E. Zur Kenntnis der Fluorescein-methyläther. Ber. Dtsch. Chem. Ges. 1913, 46, 1951–1959. [Google Scholar] [CrossRef] [Green Version]
- Marganakop, S.; Kattimani, P.; Belgur Satyanarayana, S.; Kamble, R. Microwave Synthesized Functional Dyes. In Microwave Heating—Electromagnetic Fields Causing Thermal and Non-Thermal Effects; Churyumov, G., Ed.; IntechOpen: London, UK, 2021; pp. 1–24. [Google Scholar]
- Han, J.W.; Castro, J.C.; Burgess, K. Microwave-assisted functionalization of bromo-fluorescein and bromorhodamine derivatives. Tetrahedron Lett. 2003, 44, 9359–9362. [Google Scholar] [CrossRef]
- Feng, S.; Liu, D.; Feng, W.; Feng, G. Allyl Fluorescein Ethers as Promising Fluorescent Probes for Carbon Monoxide Imaging in Living Cells. Anal. Chem. 2017, 89, 3754–3760. [Google Scholar] [CrossRef]
- Guan, X.; Lai, S.; Su, Z. Facile preparation and potential application of water-soluble polymeric temperature/pH probes bearing fluorescein. J. Appl. Polym. Sci. 2011, 122, 1968–1975. [Google Scholar] [CrossRef]
- Hanabusa, K.; Ueda, T.; Takata, S.; Suzuki, M. Synthesis of Fluorescent Gelators and Direct Observation of Gelation with a Fluorescence Microscope. Chem. A Eur. J. 2016, 22, 16939–16949. [Google Scholar] [CrossRef]
- Abdel-Latif, E.; Metwally, M.A. Waste-free solid-state organic syntheses: Solvent-free alkylation, heterocyclization, and azo-coupling reactions. Mon. Chem. 2007, 138, 771–776. [Google Scholar] [CrossRef]
- Nun, P.; Pérez, V.; Calmès, M.; Martinez, J.; Lamaty, F. Preparation of chiral amino esters by asymmetric phase-transfer catalyzed alkylations of schiff bases in a ball mill. Chem. A Eur. J. 2012, 18, 3773–3779. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Z.; Zhang, Y.; Su, W. Mechanically activated ring-opening reactions of N-acyl-1,2,3,4-tetrahydroisoquinolines derived from the synthesis of praziquantel intermediate. Tetrahedron 2015, 71, 6116–6123. [Google Scholar] [CrossRef]
- Sawyer, J.S.; Schmittling, E.A.; Palkowitz, J.A.; Smith, W.J. Synthesis of diaryl ethers, diaryl thioethers, and diarylamines mediated by potassium fluoride-alumina and 18-crown-6: Expansion of scope and utility. J. Org. Chem. 1998, 63, 6338–6343. [Google Scholar] [CrossRef]
- Klonis, N.; Sawyer, W.H. Spectral properties of the prototropic forms of fluorescein in aqueous solution. J. Fluoresc. 1996, 6, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Swamy, K.M.K.; Yoon, J. Study on various fluorescein derivatives as pH sensors. Tetrahedron Lett. 2011, 52, 2340–2343. [Google Scholar] [CrossRef]
- Jin, J.Y.; Kim, H.G.; Hong, C.H.; Suh, E.K.; Lee, Y.S. White light emission from a blue LED, combined with a sodium salt of fluorescein dye. Synth. Met. 2007, 157, 138–141. [Google Scholar] [CrossRef]
- Da Silva, B.H.S.T.; Bregadiolli, B.A.; de Oliveira Graeff, C.F.; da Silva-Filho, L.C. NbCl 5-Promoted Synthesis of Fluorescein Dye Derivatives: Spectroscopic and Spectrometric Characterization and Their Application in Dye-Sensitized Solar Cells. ChempPlusChem 2017, 82, 261–269. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Song, X.; Xia, Y.; Yu, H.; Zhang, X.; Xiao, Y.; Song, Y. Ratiometric imaging of mitochondrial pH in living cells with a colorimetric fluorescent probe based on fluorescein derivative. Sens. Actuators B Chem. 2017, 253, 58–68. [Google Scholar] [CrossRef]
- Martin, M.M.; Lindqvist, L. The pH dependence of fluorescein fluorescence. J. Lumin. 1975, 10, 381–390. [Google Scholar] [CrossRef]







| Entry | Microwave Heating Yield (%) | Classical Heating Yield (%) | Ball-Milling Yield (%) | ||
|---|---|---|---|---|---|
| A | B | A | B | B | |
| 1 (methylated derivatives) | 29 | 68 | 19 | 56 | 55 |
| 2 (ethylated derivatives) | 25 | 62 | 15 | 35 | 41 |
| Origin | Compound | λabs (nm) | λem (nm) | T (nm) | QY (%) | Concluded Form |
|---|---|---|---|---|---|---|
| Reference | Fluorescein | 437 | 475 | 3.5–4.4 | 90–100 | Cationic [4,7,42,47] |
| Fluorescein | 453; 474 | 517 | 2.97 ± 0.02 | 29 | Neutral [4,7,42,47] | |
| This work | 1B (methylated open form) | 455; 475 | 515 | 3.23 | 37.88 | Neutral |
| 2B (ethylated open form) | 452; 473 | 517 | 3.24 | 36.56 | Neutral | |
| 1A (methylated closed form) | 440 | 460 | 4.28 | 23.18 | Cationic | |
| 2A (ethylated closed form) | 440 | 460 | 3.49 | 19.89 | Cationic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdończyk, M.; Potaniec, B.; Skoreński, M.; Cybińska, J. Development of Efficient One-Pot Methods for the Synthesis of Luminescent Dyes and Sol–Gel Hybrid Materials. Materials 2022, 15, 203. https://doi.org/10.3390/ma15010203
Zdończyk M, Potaniec B, Skoreński M, Cybińska J. Development of Efficient One-Pot Methods for the Synthesis of Luminescent Dyes and Sol–Gel Hybrid Materials. Materials. 2022; 15(1):203. https://doi.org/10.3390/ma15010203
Chicago/Turabian StyleZdończyk, Maria, Bartłomiej Potaniec, Marcin Skoreński, and Joanna Cybińska. 2022. "Development of Efficient One-Pot Methods for the Synthesis of Luminescent Dyes and Sol–Gel Hybrid Materials" Materials 15, no. 1: 203. https://doi.org/10.3390/ma15010203