Self-Assembly of Asymmetrically Functionalized Titania Nanoparticles into Nanoshells
Abstract
:1. Introduction
2. Materials and Methods
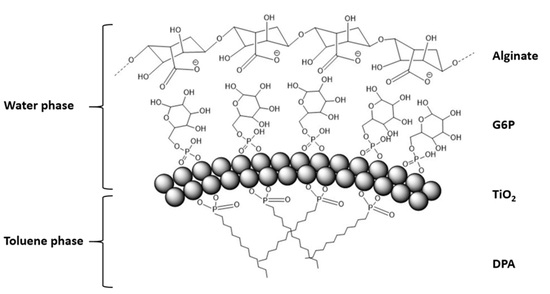
3. Results and Discussion
4. Drug Release
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A






References
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Caruso, R.A. Recent Progress in the Synthesis of Spherical Titania Nanostructures and Their Applications. Adv. Funct. Mater. 2013, 23, 1356–1374. [Google Scholar] [CrossRef]
- Kotov, N.A. Self-Assembly of Inorganic Nanoparticles: Ab ovo(a). Europhys. Lett. 2017, 119, 66008. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Wang, J.; Lv, F.; Xiao, S.; Nuckolls, C.; Hexing, L. Synthesis and Self-Assembly of Photonic Materials from Nanocrystalline Titania Sheets. J. Am. Chem. Soc. 2013, 135, 4719–4721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Wang, X.; Li, Y.; Song, B.; Bolarinwa, O.; Reese, R.A.; Zhang, T.; Wang, X.Q.; Cai, J.; et al. Supersnowflakes: Stepwise Self-Assembly and Dynamic Exchange of Rhombus Star-Shaped Supramolecules. J. Am. Chem. Soc. 2017, 139, 8174–8185. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Hao, C.; Sen, S.; Xu, C.; Král, P.; Kotov, N.A. Template-Free Hierarchical Self-Assembly of Iron Diselenide Nanoparticles into Mesoscale Hedgehogs. J. Am. Chem. Soc. 2017, 139, 16630–16639. [Google Scholar] [CrossRef]
- Glotzer, S.C.; Solomon, M.J.; Kotov, N.A. Self-Assembly: From Nanoscale to Microscale Colloids. AIChE J. 2004, 50, 2978–2985. [Google Scholar] [CrossRef]
- Jiang, W.; Qu, Z.B.; Kumar, P.; Vecchio, D.; Wang, Y.; Ma, Y.; Bahng, J.H.; Bernardino, K.; Gomes, W.R.; Colombari, F.M.; et al. Emergence of Complexity in Hierarchically Organized Chiral Particles. Science 2020, 368, 642–648. [Google Scholar] [CrossRef] [Green Version]
- Walther, A.; Müller, A.H.E. Janus Particles: Synthesis, Self-Assembly, Physical Properties, and Applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef]
- Su, H.; Price, C.A.H.; Jing, L.; Tian, Q.; Liu, J.; Qian, K. Janus Particles: Design, Preparation, and Biomedical Applications. Mater. Today Biol. 2019, 4, 100033. [Google Scholar] [CrossRef]
- Chen, Q.; Bae, S.C.; Granick, S. Directed Self-Assembly of a Colloidal Kagome Lattice. Nature 2011, 469, 381–384. [Google Scholar] [CrossRef]
- Chen, Q.; Diesel, E.; Whitmer, J.K.; Bae, S.C.; Luijten, E.; Granick, S. Triblock Colloids for Directed Self-Assembly. J. Am. Chem. Soc. 2011, 133, 7725–7727. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.D.; Kraeutler, M.J.; Yang, S.; Composto, R.J. Patchy and Multiregion Janus Particles with Tunable Optical Properties. Nano Lett. 2010, 10, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Isojima, T.; Lattuada, M.; Vander Sande, J.B.; Hatton, T.A. Reversible Clustering of pH- and Temperature-Responsive Janus Magnetic Nanoparticles. ASC Nano 2008, 2, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, Y.; Bolzinger, M.A. Emulsions Stabilized with Solid Nanoparticles: Pickering Emulsions. Colloids Surf. A Physochemical Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Harman, C.L.G.; Paterl, M.A.; Guldin, S.; Davies, G.L. Recent Developments in Pickering Emulsions for Biomedical Applications. Curr. Opin. Colloid Interface Sci. 2019, 39, 173–189. [Google Scholar] [CrossRef]
- Andala, D.M.; Shin, S.H.R.; Lee, H.Y.; Bishop, K.J.M. Templated Synthesis of Amphiphilic Nanoparticles at the Liquid-Liquid Interface. ACS Nano 2012, 6, 1044–1050. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, Z.; Zhang, L.; Shi, J.; Yang, D. Sol-Gel Derived Boehmite as an Efficient and Robust Carrier for Enzyme Encapsulation. Ind. Eng. Chem. Res. 2012, 51, 255–261. [Google Scholar] [CrossRef]
- Sakkos, J.K.; Mutlu, B.R.; Wackett, L.P.; Aksan, A. Adsorption and Biodegradation of Aromatic Chemicals by Bacteria Encapsulated in a Hydrophobic Silica Gel. ACS Appl. Mater. Interfaces 2017, 9, 26848–26858. [Google Scholar] [CrossRef]
- Amoura, M.; Nassif, N.; Roux, C.; Livage, J.; Coradin, T. Sol-Gel Encapsulation of Cells is Not Limited to Silica: Long-Term Viability of Bacteria in Alumina Matrices. Chem. Commun. 2007, 39, 4015–4017. [Google Scholar] [CrossRef]
- Kessler, V.G.; Seisenbaeva, G.A.; Unell, M.; Håkansson, S. Chemically Triggered Biodelivery Using Metal-Organic Sol-Gel Synthesis. Angew. Chem. Int. Ed. 2008, 47, 8506–8509. [Google Scholar] [CrossRef] [PubMed]
- Youn, W.; Ko, E.H.; Kim, M.H.; Park, M.; Hong, D.; Seisenbaeva, G.A.; Kessler, V.G.; Choi, I.S. Cytoprotective Encapsulation of Individual Jurkat T Cells within Durable TiO2 Shells for T-Cell Therapy. Angew. Chem. Int. Ed. 2017, 56, 10702–10706. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Wahi, R.; Kurian, P.A.; Liu, Y.; West, J.L.; Ausman, K.D.; Warheit, D.B.; Colvin, V.L. Correlating Nanoscale Titania Structure with Toxicity: A Cytotoxicity and Inflammatory Response Study with Human Dermal Fibroblasts and Human Lung Epithelial Cells. Toxicol. Sci. 2006, 92, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Ekstrand-Hammarström, B.; Hong, J.; Davoodpour, P.; Sandholm, K.; Ekdahl, K.N.; Bucht, A.; Nilsson, B. TiO2 Nanoparticles Tested in a Novel Screening Whole Human Blood Model of Toxicity Trigger Adverse Activation of the Kallikrein System at Low Concentrations. Biomaterials 2015, 51, 58–68. [Google Scholar] [CrossRef]
- Pang, H.; Yang, H.; Guo, C.X.; Lu, J.; Li, M. Nanoparticle Self-Assembled Hollow TiO2 Spheres with Well Matching Visible Light Scattering for High Performance Dye-Sensitized Solar Cells. Chem. Commun. 2012, 48, 8832–8834. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Moloney, M.P.; Tekoriute, R.; Hardy-Dessource, A.; Nedelec, J.M.; Gun’ko, Y.K.; Kessler, V.G. Biomimetic Synthesis of Hierarchically Porous Metal Oxide Microparticles—Potential Scaffolds for Drug Delivery and Catalysis. Langmuir 2010, 26, 9809–9817. [Google Scholar] [CrossRef]
- Chen, T.; Clover, P.J.; Bon, S.A.F. Organic-Inorganic Hybrid Hollow Spheres Prepared from TiO2-Stabilized Pickering Emulsion Polymerization. Adv. Mater. 2007, 19, 2286–2289. [Google Scholar] [CrossRef]
- Li, S.; Wang, F.; Dai, H.; Jiang, X.; Ye, C.; Min, J. Self-Assembly of Silica Nanoparticles into Hollow Spheres via a Microwave-Assisted Aerosol Process. Mater. Res. Bull. 2016, 74, 459–464. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.; Yang, X.L.; Xu, H.B. Controllable Synthesis of Hollow Mesoporous Silica Nanoparticles Templated by Kinetic Self-Assembly Using a Gemini Surfactant. RSC Adv. 2013, 3, 16304. [Google Scholar] [CrossRef]
- Nilsing, M.; Lunell, S.; Persson, P.; Ojamae, L. Phosphonic Acid Adsorption at the TiO2 Anatase (101) Surface Investigated by Periodic Hybrid HF-DFT Computations. Surf. Sci. 2005, 582, 49–60. [Google Scholar] [CrossRef]
- Nilsing, M.; Persson, P.; Ojamae, L. Anchor Group Influence on Molecule-Metal Oxide Interfaces: Periodic Hybrid DFT Study of Pyridine Bound to TiO2 via Carboxylic and Phosphonic Acid. Chem. Phys. Lett. 2005, 415, 375–380. [Google Scholar] [CrossRef]
- Svensson, F.G.; Daniel, G.; Tai, C.W.; Seisenbaeva, G.A.; Kessler, V.G. Titanium Phosphonate Oxo-Alkoxide “Clusters”: Solution Stability and Facile Hydrolytic Transformation into Nano Titania. RSC Adv. 2020, 10, 6873–6883. [Google Scholar] [CrossRef]
- Azouani, R.; Soloviev, A.; Benmami, M.; Chhor, K.; Bocquet, J.F.; Kanaev, A. Stability and Growth of Titanium-Oxo-Alkoxy TixOy(OiPr)z Clusters. J. Phys. Chem. C 2007, 111, 16243–16248. [Google Scholar] [CrossRef]
- Xia, Y.; Nguyen, T.D.; Yang, M.; Lee, B.; Santos, A.; Podsiadlo, P.; Tang, Z.; Glotzer, S.C.; Kotov, N.A. Self-Assembly of Self-Limiting Monodisperse Supraparticles from Polydisperse Nanoparticles. Nat. Nanotechnol. 2012, 7, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccinini, E.; Pallarola, D.; Battaglini, F.; Azzaroni, O. Self-Limited Self-Assembly of Nanoparticles into Supraparticles: Towards Supramolecular Colloidal Materials by Design. Mol. Syst. Des. Eng. 2016, 1, 155–162. [Google Scholar] [CrossRef]
- Evdokimova, O.L.; Svensson, F.G.; Agafonov, A.V.; Håkansson, S.; Seisenbaeva, G.A.; Kessler, V.G. Hybrid Drug Delivery Patches Based on Spherical Cellulose Nanocrystals and Colloid Titania-Synthesis and Antibacterial Properties. Nanomaterials 2018, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Kulak, A.; Hall, S.R.; Mann, S. Single-Step Fabrication of Drug-Encapsulated Inorganic Microspheres with Complex Form by Sonication-Induced Nanoparticle Assembly. Chem. Commun. 2004, 5, 576–577. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svensson, F.G.; Seisenbaeva, G.A.; Kotov, N.A.; Kessler, V.G. Self-Assembly of Asymmetrically Functionalized Titania Nanoparticles into Nanoshells. Materials 2020, 13, 4856. https://doi.org/10.3390/ma13214856
Svensson FG, Seisenbaeva GA, Kotov NA, Kessler VG. Self-Assembly of Asymmetrically Functionalized Titania Nanoparticles into Nanoshells. Materials. 2020; 13(21):4856. https://doi.org/10.3390/ma13214856
Chicago/Turabian StyleSvensson, Fredric G., Gulaim A. Seisenbaeva, Nicholas A. Kotov, and Vadim G. Kessler. 2020. "Self-Assembly of Asymmetrically Functionalized Titania Nanoparticles into Nanoshells" Materials 13, no. 21: 4856. https://doi.org/10.3390/ma13214856