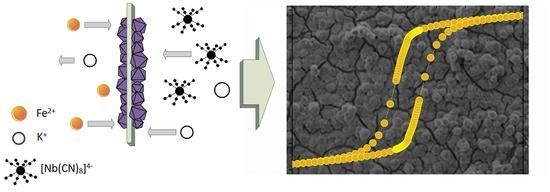
Magnetic, Structural and Spectroscopic Properties of Iron(II)-Octacyanoniobate(IV) Crystalline Film Obtained by Ion-Exchange Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Synthesis
2.2. Characterization Techniques
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, W.; Hong, Y.; Le, Z.; Li, Q.; Li, W.; Hu, M. Synthesis of coordination polymer thin films with conductance-response to mechanical stimulation. Chem. Commun. 2019, 55, 2545–2548. [Google Scholar] [CrossRef]
- Verdaguer, M.; Girolami, G.S. Magnetic Prussian Blue Analogs. In Magnetism: Molecules to Materials; Wiley–VCH & KGaA: Weinheim, Germany, 2003; Volume 5, pp. 283–346. ISBN 9783527620548. [Google Scholar]
- Verdaguer, M.; Bleuzen, A.; Marvaud, V.; Vaissermann, J.; Seuleiman, M.; Desplanches, C.; Scuiller, A.; Train, C.; Garde, R.; Gelly, G.; et al. Molecules to build solids: High TC molecule-based magnets by design and recent revival of cyano complexes chemistry. Coord. Chem. Rev. 1999, 190–192, 1023–1047. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Fujishima, A.; Hashimoto, K. Transparent and Colored Magnetic Thin Films: (FeIIx CrII1−x)1.5 [CrIII (CN)6]. J. Am. Chem. Soc. 1998, 120, 5349–5350. [Google Scholar] [CrossRef]
- Coronado, E.; Makarewicz, M.; Prieto-Ruiz, J.P.; Prima-García, H.; Romero, F.M. Magneto-optical properties of electrodeposited thin films of the molecule-based magnet Cr5.5(CN)12·11.5H2O. Adv. Mater. 2011, 23, 4323–4326. [Google Scholar] [CrossRef]
- Hedley, L.; Robertson, N.; Johansson, J.O. Electrochromic Thin Films of the V-Cr Prussian Blue Analogue Molecular Magnet. Electrochim. Acta 2017, 236, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Clemente-León, M.; Coronado, E.; López-Muñoz, Á.; Repetto, D.; Mingotaud, C.; Brinzei, D.; Catala, L.; Mallah, T. Magnetic Langmuir-Blodgett films of bimetallic coordination nanoparticles of Cs0.4Ni[Cr(CN)6]0.9. Chem. Mater. 2008, 20, 4642–4652. [Google Scholar] [CrossRef]
- Yamamoto, T.; Umemura, Y.; Sato, O.; Einaga, Y. Photomagnetic Langmuir–Blodgett films consisting of azobenzene and Prussian Blue: Correlation between the film structure and the photomagnetic efficiency. Sci. Technol. Adv. Mater. 2006, 7, 134–138. [Google Scholar] [CrossRef]
- Fitta, M.; Prima-Garcia, H.; Czaja, P.; Korzeniak, T.; Krupiński, M.; Wojtyniak, M.; Bałanda, M. Magnetic and magneto-optical properties of nickel hexacyanoferrate/chromate thin films. RSC Adv. 2017, 7, 1382–1386. [Google Scholar] [CrossRef] [Green Version]
- Pajerowski, D.M.; Gardner, J.E.; Talham, D.R.; Meisel, M.W. Anisotropic magnetism in Prussian blue analogue films. New J. Chem. 2011, 35, 1320. [Google Scholar] [CrossRef]
- Tozawa, M.; Ohkoshi, S.I.; Kojima, N.; Hashimoto, K. Ion-exchange synthesis and magneto-optical spectra of colored magnetic thin films composed of metal(ii) hexacyanochromate(iii). Chem. Commun. 2003, 3, 1204–1205. [Google Scholar] [CrossRef] [PubMed]
- Volatron, F.; Heurtaux, D.; Catala, L.; Mathonière, C.; Gloter, A.; Stéphan, O.; Repetto, D.; Clemente-León, M.; Coronado, E.; Mallah, T. Photo-induced magnetic bistability in a controlled assembly of anisotropic coordination nanoparticles. Chem. Commun. 2011, 47, 1985–1987. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, B.; Bałanda, M.; Reczyński, M.; Majcher, A.M.; Kozieł, M.; Nitek, W.; Łasocha, W.; Sieklucka, B. A water sensitive ferromagnetic [Ni(cyclam)]2[Nb(CN)8] network. Dalt. Trans. 2013, 42, 2616–2621. [Google Scholar] [CrossRef]
- Ohkoshi, S.-I.; Namai, A.; Tokoro, H. Humidity sensitivity, organic molecule sensitivity, and superionic conductivity on porous magnets based on cyano-bridged bimetal assemblies. Coord. Chem. Rev. 2019, 380, 572–583. [Google Scholar] [CrossRef]
- Ozaki, N.; Tokoro, H.; Hamada, Y.; Namai, A.; Matsuda, T.; Kaneko, S.; Ohkoshi, S. Photoinduced Magnetization with a High Curie Temperature and a Large Coercive Field in a Co-W Bimetallic Assembly. Adv. Funct. Mater. 2012, 22, 2089–2093. [Google Scholar] [CrossRef]
- Magott, M.; Reczyński, M.; Gaweł, B.; Sieklucka, B.; Pinkowicz, D. A Photomagnetic Sponge: High-Temperature Light-Induced Ferrimagnet Controlled by Water Sorption. J. Am. Chem. Soc. 2018, 140, 15876–15882. [Google Scholar] [CrossRef] [PubMed]
- Fitta, M.; Pełka, R.; Konieczny, P.; Bałanda, M. Multifunctional molecular magnets: Magnetocaloric effect in octacyanometallates. Crystals 2019, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Herrera, J.M.; Franz, P.; Podgajny, R.; Pilkington, M.; Biner, M.; Decurtins, S.; Stoeckli-Evans, H.; Neels, A.; Garde, R.; Dromzée, Y.; et al. Three-dimensional bimetallic octacyanidometalates [MIV{(μ-CN)4MnII(H2O)2}2·4H2O]n (M=Nb, Mo, W): Synthesis, single-crystal X-ray diffraction and magnetism. Comptes Rendus Chim. 2008, 11, 1192–1199. [Google Scholar] [CrossRef]
- Wei, R.-M.; Cao, F.; Li, J.; Yang, L.; Han, Y.; Zhang, X.-L.; Zhang, Z.; Wang, X.-Y.; Song, Y. Single-Chain Magnets Based on Octacyanotungstate with the Highest Energy Barriers for Cyanide Compounds. Sci. Rep. 2016, 6, 24372. [Google Scholar] [CrossRef]
- Nakagawa, K.; Imoto, K.; Miyahara, H.; Ohkoshi, S.I. Syntheses, crystal structures, and magnetic properties of cyano-bridged Mn(II)-Nb(IV) bimetal assemblies. Polyhedron 2013, 52, 424–428. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhang, Y.Z.; Southerland, H.; Prosvirin, A.V.; Zhao, H.; Dunbar, K.R. Variations in topology and magnetic properties of hepta- and octacyanometallates of molybdenum with manganese(ii). Dalt. Trans. 2014, 43, 6802–6810. [Google Scholar] [CrossRef] [PubMed]
- Pinkowicz, D.; Podgajny, R.; Pełka, R.; Nitek, W.; Bałanda, M.; Makarewicz, M.; Czapla, M.; Zukrowski, J.; Kapusta, C.; Zajc, D.; et al. Iron(II)-octacyanoniobate(IV) ferromagnet with TC 43 K. Dalt. Trans. 2009, 7771–7777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handzlik, G.; Magott, M.; Sieklucka, B.; Pinkowicz, D. Alternative synthetic route to potassium octacyanidoniobate(IV) and its molybdenum congener. Eur. J. Inorg. Chem. 2016, 30, 4872–4877. [Google Scholar] [CrossRef]
- Maiti, J.; Kakati, N.; Woo, S.P.; Yoon, Y.S. Nafion® based hybrid composite membrane containing GO and dihydrogen phosphate functionalized ionic liquid for high temperature polymer electrolyte membrane fuel cell. Compos. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Boutsika, L.G.; Enotiadis, A.; Nicotera, I.; Simari, C.; Charalambopoulou, G.; Giannelis, E.P.; Steriotis, T. Nafion® nanocomposite membranes with enhanced properties at high temperature and low humidity environments. Int. J. Hydrogen Energy 2016, 155, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Yusuf, S.M.; Keller, L.; Yakhmi, J.V.; Srivastava, J.K.; Paulose, P.L. Variation of structural and magnetic properties with composition in the (Cox Ni1-x)1.5 [Fe (CN)6] •z H2O series. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 75, 224419. [Google Scholar] [CrossRef] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sas, W.; Pinkowicz, D.; Perzanowski, M.; Fitta, M. Magnetic, Structural and Spectroscopic Properties of Iron(II)-Octacyanoniobate(IV) Crystalline Film Obtained by Ion-Exchange Synthesis. Materials 2020, 13, 3029. https://doi.org/10.3390/ma13133029
Sas W, Pinkowicz D, Perzanowski M, Fitta M. Magnetic, Structural and Spectroscopic Properties of Iron(II)-Octacyanoniobate(IV) Crystalline Film Obtained by Ion-Exchange Synthesis. Materials. 2020; 13(13):3029. https://doi.org/10.3390/ma13133029
Chicago/Turabian StyleSas, Wojciech, Dawid Pinkowicz, Marcin Perzanowski, and Magdalena Fitta. 2020. "Magnetic, Structural and Spectroscopic Properties of Iron(II)-Octacyanoniobate(IV) Crystalline Film Obtained by Ion-Exchange Synthesis" Materials 13, no. 13: 3029. https://doi.org/10.3390/ma13133029