Galvanically Stimulated Degradation of Carbon-Fiber Reinforced Polymer Composites: A Critical Review
Abstract
:1. Introduction
2. Composition of Carbon-Fiber Reinforced Polymers (CFRPs)
3. The Nature of the Carbon–Epoxy Matrix Interface in Carbon-fiber reinforced polymers (CFRPs) and Its Effects on Composite Properties.
4. Efforts at Modification of CFRPs with Nanofillers and Possible Consequences for Conductivity and Galvanic Corrosion of Coupled Metals.
5. The Driving Force for Galvanically Stimulated Degradation of Carbon Fiber Reinforced Polymer Composites
6. Mechanism of Oxygen Reduction on Carbon Electrodes
7. The Surface Chemistry of Carbon and Its Effects on Cathodic Processes on CFRPs.
8. Carbon-Fiber Reinforced Polymers (CFRPs) Degradation under Cathodic Polarization.
9. Chemical Stability/Degradation of the Polymer Matrix
10. CFRP Degradation under Anodic Polarization
11. Monitoring and Mitigating Degradation in Carbon Fiber Reinforced Composites.
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mallick, P.K. Fibre-Reinforced Composites: Materials, Manufacturing, and Design; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Scholz, M.S.; Blanchfield, J.P.; Bloom, L.D.; Coburn, B.H.; Elkington, M.; Fuller, J.D.; Gilbert, M.E.; Muflahi, S.A.; Pernice, M.F.; Rae, S.I.; et al. The use of composite materials in modern orthopaedic medicine and prosthetic devices: A review. Compos. Sci. Technol. 2011, 71, 1791–1803. [Google Scholar] [CrossRef]
- Williams, D.F.; McNamara, A.; Turner, R.M. Potential of polyetheretherketone (PEEK) and carbon-fibre-reinforced PEEK in medical applications. J. Mater. Sci. Lett. 1987, 6, 188–190. [Google Scholar] [CrossRef]
- Scotchford, C.A.; Garle, M.J.; Batchelor, J.; Bradley, J.; Grant, D.M. Use of a novel carbon fibre composite material for the femoral stem component of a THR system: In vitro biological assessment. Biomaterials 2003, 24, 4871–4879. [Google Scholar] [PubMed]
- Saito, N.; Aoki, K.; Usui, Y.; Shimizu, M.; Hara, K.; Narita, N.; Ogihara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Application of carbon fibres to biomaterials: A new era of nano-level control of carbon fibres after 30-years of development. Chem. Soc. Rev. 2011, 40, 3824–3834. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, E.L.; Rath, E.; Shlaifer, A.; Chechik, O.; Maman, E.; Salai, M. Carbon fibre reinforced PEEK Optima—A composite material biomechanical properties and wear/debris characteristics of CF-PEEK composites for orthopedic trauma implants. J. Mech. Behav. Biomed. Mater. 2013, 17, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, I.; Takao, M.; Bandoh, S.; Bertollo, N.; Walsh, W.R.; Sugano, N. In vivo implant fixation of carbon fibre-reinforced PEEK hip prostheses in an ovine model. J. Orthop. Res. 2013, 31, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xiong, G.; Yang, Z.; Raman, S.R.; Li, Q.; Ma, C.; Wang, Z.; Wan, Y. Preparation of three-dimensional braided carbon fibre-reinforced PEEK composites for potential load-bearing bone fixations. Part I. Mechanical properties and cytocompatibility. J. Mech. Behav. Biomed. Mater. 2014, 29, 103–113. [Google Scholar] [CrossRef]
- Mano, J.F.; Sousa, R.A.; Boesel, L.F.; Neves, N.M.; Reis, R.L. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: State of the art and recent developments. Compos. Sci. Technol. 2004, 64, 789–817. [Google Scholar] [CrossRef]
- Brooks, R.A.; Jones, E.; Storer, A.; Rushton, N. Biological evaluation of carbon-fibre-reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials 2004, 25, 3429–3438. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.M.; Mehats, A.; Elcocks, M.; Rushton, N.; Field, R.E.; Jones, E. Pre-clinical studies to validate the MITCH PCR™ Cup: A flexible and anatomically shaped acetabular component with novel bearing characteristics. J. Mater. Sci. Mater. Med. 2008, 19, 1729–1736. [Google Scholar] [CrossRef]
- Bagheri, Z.S.; El Sawi, I.; Schemitsch, E.H.; Zdero, R.; Bougherara, H. Biomechanical properties of an advanced new carbon/flax/epoxy composite material for bone plate applications. J. Mech. Behav. Biomed. Mater. 2013, 20, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Feerick, E.M.; Kennedy, J.; Mullett, H.; FitzPatrick, D.; McGarry, P. Investigation of metallic and carbon fibre PEEK fracture fixation devices for three-part proximal humeral fractures. Med. Eng. Phys. 2013, 35, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Vannabouathong, C.; Sprague, S.; Bhandari, M. The use of carbon-fibre-reinforced (CFR) PEEK material in orthopedic implants: A systematic review. Clinical medicine insights. Arthritis Musculoskelet. Disord. 2015, 8, 33. [Google Scholar]
- Zhang, X.; Zhang, Y.; Zhang, X.; Wang, Y.; Wang, J.; Lu, M.; Li, H. Mechanical properties and cytocompatibility of carbon fibre reinforced nano-hydroxyapatite/polyamide66 ternary biocomposite. J. Mech. Behav. Biomed. Mater. 2015, 42, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Ronca, D.; Russo, T.; D’Amora, U.; Chierchia, M.; De Santis, R.; Nicolais, L.; Ambrosio, L. Technical features and criteria in designing fibre-reinforced composite materials: From the aerospace and aeronautical field to biomedical applications. J. Appl. Biomater. Biomech. 2011, 9, 151–163. [Google Scholar] [PubMed]
- Meier, U. Carbon fibre-reinforced polymers: Modern materials in bridge engineering. Struct. Eng. Int. 1992, 2, 7–12. [Google Scholar] [CrossRef]
- Meier, U. Strengthening of structures using carbon fibre/epoxy composites. Constr. Build. Mater. 1995, 9, 341–351. [Google Scholar] [CrossRef]
- Yue, Q. Present status of research and application of strengthening and repairing technology with carbon fibre reinforced plastics (CFRP) and its outlook in China. Ind. Constr. 2000, 10, 004. [Google Scholar]
- Yue, Q. New Technology and Application of Carbon Fiber Reinforced Plastics on Strengthening & Repairing Concrete Structures. Hi-Tech Fiber Appl. 1998, 5, 1–6. [Google Scholar]
- Shrive, N.G. The use of fibre reinforced polymers to improve seismic resistance of masonry. Constr. Build. Mater. 2006, 20, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Malvar, L.J.; Cox, J.V.; Cochran, K.B. Bond between carbon fibre reinforced polymer bars and concrete. I: Experimental study. J. Compos. Constr. 2003, 7, 154–163. [Google Scholar] [CrossRef]
- Carolin, A. Carbon Fibre Reinforced Polymers for Strengthening of Structural Elements. Ph.D. Thesis, Luleå Tekniska Universitet, Luleå, Sweden, 2003. [Google Scholar]
- Lee, C.; Bonacci, J.F.; Thomas, M.D.; Maalej, M.; Khajehpour, S.; Hearn, N.; Pantazopoulou, S.; Sheikh, S. Accelerated corrosion and repair of reinforced concrete columns using carbon fibre reinforced polymer sheets. Can. J. Civ. Eng. 2000, 27, 941–948. [Google Scholar] [CrossRef]
- El-Hacha, R.; Wight, R.G.; Green, M.F. Prestressed fibre-reinforced polymer laminates for strengthening structures. Prog. Struct. Eng. Mater. 2001, 3, 111–121. [Google Scholar] [CrossRef]
- Pendhari, S.S.; Kant, T.; Desai, Y.M. Application of polymer composites in civil construction: A general review. Compos. Struct. 2008, 84, 114–124. [Google Scholar] [CrossRef]
- Benmokrane, B.; Zhang, B.; Laoubi, K.; Tighiouart, B.; Lord, I. Mechanical and bond properties of new generation of carbon fibre reinforced polymer reinforcing bars for concrete structures. Can. J. Civ. Eng. 2002, 29, 338–343. [Google Scholar] [CrossRef]
- Hollaway, L.C.; Cadei, J. Progress in the technique of upgrading metallic structures with advanced polymer composites. Prog. Struct. Eng. Mater. 2002, 4, 131–148. [Google Scholar] [CrossRef]
- Mara, V.; Haghani, R.; Harryson, P. Bridge decks of fibre reinforced polymer (FRP): A sustainable solution. Constr. Build. Mater. 2014, 50, 190–199. [Google Scholar] [CrossRef]
- Seica, M.V.; Packer, J.A. FRP materials for the rehabilitation of tubular steel structures, for underwater applications. Compos. Struct. 2007, 80, 440–450. [Google Scholar] [CrossRef]
- El-Salakawy, E.; Benmokrane, B.; Desgagné, G. Fibre-reinforced polymer composite bars for the concrete deck slab of Wotton Bridge. Can. J. Civ. Eng. 2003, 30, 861–870. [Google Scholar] [CrossRef]
- Badawi, M.; Soudki, K. Control of corrosion-induced damage in reinforced concrete beams using carbon fibre-reinforced polymer laminates. J. Compos. Constr. 2005, 9, 195–201. [Google Scholar] [CrossRef]
- Meier, U. Carbon fibre reinforced polymer cables: Why? Why not? What if? Arab. J. Sci. Eng. 2012, 37, 399–411. [Google Scholar] [CrossRef]
- Meier, U.; Brönnimann, R.; Anderegg, P.; Terrasi, G.P. 20 Years of Experience with Structural Health Monitoring of Objects with CFRP Components. In Nondestructive Testing of Materials and Structures; Springer: Dordrecht, The Netherlands, 2013; pp. 959–976. [Google Scholar]
- Hensher, D.A. Fibre-Reinforced-Plastic (FRP) Reinforcement for Concrete Structures: Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2016; Volume 42. [Google Scholar]
- Thomassin, J.M.; Jerome, C.; Pardoen, T.; Bailly, C.; Huynen, I.; Detrembleur, C. Polymer/carbon based composites as electromagnetic interference (EMI) shielding materials. Mater. Sci. Eng. R: Rep. 2013, 74, 211–232. [Google Scholar] [CrossRef]
- Wong, K.H.; Pickering, S.J.; Rudd, C.D. Recycled carbon fibre reinforced polymer composite for electromagnetic interference shielding. Compos. Part A Appl. Sci. Manuf. 2010, 41, 693–702. [Google Scholar] [CrossRef]
- Luo, X.; Chung, D.D.L. Electromagnetic interference shielding using continuous carbon-fibre carbon-matrix and polymer-matrix composites. Compos. Part B Eng. 1999, 30, 227–231. [Google Scholar] [CrossRef]
- Glatkowski, P.J.; Landis, D.H.; Piche, J.W.; Conroy, J.L.; Eikos, Inc. Carbon Nanotube Fibre-Reinforced Composite Structures for EM and Lightning Strike Protection. U.S. Patent 6,986,853, 13 April 2006. [Google Scholar]
- Mcconnell, V.P. Application of composites in sporting goods. Compr. Compos. Mater. 2000, 6, 787–809. [Google Scholar]
- Mouritz, A.P.; Gellert, E.; Burchill, P.; Challis, K. Review of advanced composite structures for naval ships and submarines. Compos. Struct. 2001, 53, 21–42. [Google Scholar] [CrossRef]
- Marsh, G. Reinforced plastics prevail on the waterfront. Reinf. Plast. 2002, 46, 3034–3235. [Google Scholar] [CrossRef]
- Ross, C.T. A conceptual design of an underwater vehicle. Ocean Eng. 2006, 33, 2087–2104. [Google Scholar] [CrossRef]
- Davies, P. Environmental degradation of composites for marine structures: New materials and new applications. Phil. Trans. R. Soc. A 2016, 374, 20150272. [Google Scholar] [CrossRef]
- Alexander, C.; Ochoa, O.O. Extending onshore pipeline repair to offshore steel risers with carbon–fibre reinforced composites. Compos. Struct. 2010, 92, 499–507. [Google Scholar] [CrossRef]
- Ochoa, O.O.; Salama, M.M. Offshore composites: Transition barriers to an enabling technology. Compos. Sci. Technol. 2005, 65, 2588–2596. [Google Scholar] [CrossRef]
- Kim, W.K.; Ochoa, O.O.; Miller, C.A. Axial and Burst Analysis of Offshore Composite Risers. In Proceedings of the 20th Annual Technical Conference of the American Society for Composites, Philadelphia, PA, USA, 7–9 September 2005. [Google Scholar]
- Kruijer, M.P.; Warnet, L.L.; Akkerman, R. Analysis of the mechanical properties of a reinforced thermoplastic pipe (RTP). Compos. Part A Appl. Sci. Manuf. 2005, 36, 291–300. [Google Scholar] [CrossRef]
- Fisher, E.H.; Gibson, A.G. Continuous fibre reinforced thermoplastic pipes for transport and distribution of fluids for the oil and gas industries. Plast. Rubber Compos. Process. Appl. 1998, 27, 447–451. [Google Scholar]
- Ainsworth, L. Fibre-reinforced plastic pipes and applications. Composites 1981, 12, 185–190. [Google Scholar] [CrossRef]
- Price, J.C. The “State of the Art” in composite material development and applications for the oil and gas industry. In Proceedings of the Twelfth International Offshore and Polar Engineering Conference, Kitakyushu, Japan, 26–31 May 2002. [Google Scholar]
- Williams, G.; Trask, R.; Bond, I. A self-healing carbon fibre reinforced polymer for aerospace applications. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1525–1532. [Google Scholar] [CrossRef]
- Hayes, S.A.; Zhang, W.; Branthwaite, M.; Jones, F.R. Self-healing of damage in fibre-reinforced polymer-matrix composites. J. R. Soc. Interface 2007, 4, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, R.; Davila, M.M.; Elizalde, M.P.; Mattusch, J.; Wennrich, R. Capability of a carbon–polyvinylchloride composite electrode for the detection of dopamine, ascorbic acid and uric acid. Electrochim. Acta 2004, 49, 851–859. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.W. Carbon nanotube networks: Sensing of distributed strain and damage for life prediction and self healing. Adv. Mater. 2006, 18, 2837–2841. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.W. Real-time in situ sensing of damage evolution in advanced fibre composites using carbon nanotube networks. Nanotechnology 2008, 19, 215713. [Google Scholar] [CrossRef]
- Tekinalp, H.L.; Kunc, V.; Velez-Garcia, G.M.; Duty, C.E.; Love, L.J.; Naskar, A.K.; Blue, C.A.; Ozcan, S. Highly oriented carbon fibre–polymer composites via additive manufacturing. Compos. Sci. Technol. 2014, 105, 144–150. [Google Scholar] [CrossRef]
- Hofstatter, T.; Pedersen, D.B.; Tosello, G.; Hansen, H.N. Applications of Fibre-Reinforced Polymers in Additive Manufacturing. Procedia CIRP 2017, 66, 312–316. [Google Scholar] [CrossRef]
- Ning, F.; Cong, W.; Qiu, J.; Wei, J.; Wang, S. Additive manufacturing of carbon fibre reinforced thermoplastic composites using fused deposition modeling. Compos. Part B Eng. 2015, 80, 369–378. [Google Scholar] [CrossRef]
- Ning, F.; Cong, W.; Hu, Y.; Wang, H. Additive manufacturing of carbon fibre-reinforced plastic composites using fused deposition modeling: Effects of process parameters on tensile properties. J. Compos. Mater. 2017, 51, 451–462. [Google Scholar] [CrossRef]
- Van Der Klift, F.; Koga, Y.; Todoroki, A.; Ueda, M.; Hirano, Y.; Matsuzaki, R. 3D printing of continuous carbon fibre reinforced thermo-plastic (CFRTP) tensile test specimens. Open J. Compos. Mater. 2015, 6, 18. [Google Scholar] [CrossRef]
- Invernizzi, M.; Natale, G.; Levi, M.; Turri, S.; Griffini, G. UV-assisted 3D printing of glass and carbon fibre-reinforced dual-cure polymer composites. Materials 2016, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, R.; Ueda, M.; Namiki, M.; Jeong, T.K.; Asahara, H.; Horiguchi, K.; Nakamura, T.; Todoroki, A.; Hirano, Y. Three-dimensional printing of continuous-fibre composites by in-nozzle impregnation. Sci. Rep. 2016, 6, 23058. [Google Scholar] [CrossRef]
- Lewicki, J.P.; Rodriguez, J.N.; Zhu, C.; Worsley, M.A.; Wu, A.S.; Kanarska, Y.; Horn, J.D.; Duoss, E.B.; Ortega, J.M.; Elmer, W.; et al. 3D-Printing of Meso-structurally Ordered Carbon Fibre/Polymer Composites with Unprecedented Orthotropic Physical Properties. Sci. Rep. 2017, 7, 43401. [Google Scholar] [CrossRef]
- Quan, Z.; Wu, A.; Keefe, M.; Qin, X.; Yu, J.; Suhr, J.; Byun, J.H.; Kim, B.S.; Chou, T.W. Additive manufacturing of multi-directional preforms for composites: Opportunities and challenges. Mater. Today 2015, 18, 503–512. [Google Scholar] [CrossRef]
- Marsh, G. Composites strengthen aerospace hold. Reinf. Plast. 2002, 46, 40–43. [Google Scholar] [CrossRef]
- Marsh, G. A new start for marine propellers? Reinf. Plast. 2004, 48, 34–38. [Google Scholar] [CrossRef]
- Marsh, G. Airframers exploit composites in battle for supremacy. Reinf. Plast. 2005, 49, 26–32. [Google Scholar] [CrossRef]
- Marsh, G. Next step for automotive materials. Mater. Today 2003, 6, 36–43. [Google Scholar] [CrossRef]
- Marsh, G. Composites lift off in primary aerostructures. Reinf. Plast. 2004, 48, 22–27. [Google Scholar] [CrossRef]
- Marsh, G. Composites get in deep with new-generation engine. Reinf. Plast. 2006, 50, 26–29. [Google Scholar] [CrossRef]
- Marsh, G. Duelling with composites. Reinf. Plast. 2006, 50, 18–23. [Google Scholar] [CrossRef]
- Marsh, G. Airbus takes on Boeing with reinforced plastic A350 XWB. Reinf. Plast. 2007, 51, 26–29. [Google Scholar] [CrossRef]
- Marsh, G. Airbus A350 XWB update. Reinf. Plast. 2010, 54, 20–24. [Google Scholar] [CrossRef]
- Marsh, G. Marine composites—Drawbacks and successes. Reinf. Plast. 2010, 54, 18–22. [Google Scholar] [CrossRef]
- Marsh, G. Bombardier throws down the gauntlet with CSeries airliner. Reinf. Plast. 2011, 55, 22–26. [Google Scholar] [CrossRef]
- Marsh, G. Aero engines lose weight thanks to composites. Reinf. Plast. 2012, 56, 32–35. [Google Scholar] [CrossRef]
- Mrazova, M. Advanced composite materials of the future in aerospace industry. Incas Bull. 2013, 5, 139. [Google Scholar]
- Marsh, G. Composites and metals—A marriage of convenience? Reinf. Plast. 2014, 58, 38–42. [Google Scholar] [CrossRef]
- Marsh, G. Composites flying high. Reinf. Plast. 2014, 58, 14–18. [Google Scholar] [CrossRef]
- Marsh, G. Reinforced thermoplastics, the next wave? Reinf. Plast. 2014, 58, 24–28. [Google Scholar] [CrossRef]
- Marsh, G. Composites in commercial jets. Reinf. Plast. 2015, 59, 190–193. [Google Scholar] [CrossRef]
- Kolesnikov, B.; Herbeck, L.; Fink, A. CFRP/titanium hybrid material for improving composite bolted joints. Compos. Struct. 2008, 83, 368–380. [Google Scholar] [CrossRef]
- Kradinov, V.; Madenci, E.; Ambur, D.R. Bolted lap joints of laminates with varying thickness and metallic inserts. Compos. Struct. 2005, 68, 75–85. [Google Scholar] [CrossRef]
- Kradinov, V.; Madenci, E.; Ambur, D.R. Combined in-plane and through-the-thickness analysis for failure prediction of bolted composite joints. Compos. Struct. 2007, 77, 127–147. [Google Scholar] [CrossRef] [Green Version]
- Martinsen, K.; Hu, S.J.; Carlson, B.E. Joining of dissimilar materials. Cirp Ann.-Manuf. Technol. 2015, 64, 679–699. [Google Scholar] [CrossRef] [Green Version]
- Möller, F.; Thomy, C.; Vollertsen, F.; Schiebel, P.; Hoffmeister, C.; Herrmann, A.S. Novel method for joining CFRP to aluminium. Phys. Procedia 2010, 5, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Fink, A.; Camanho, P.P.; Andrés, J.M.; Pfeiffer, E.; Obst, A. Hybrid CFRP/titanium bolted joints: Performance assessment and application to a spacecraft payload adaptor. Compos. Sci. Technol. 2010, 70, 305–317. [Google Scholar] [CrossRef]
- Balle, F.; Wagner, G.; Eifler, D. Ultrasonic spot welding of aluminum sheet/carbon fibre reinforced polymer–joints. Mater. Werkst. 2007, 38, 934–938. [Google Scholar] [CrossRef]
- Ucsnik, S.; Scheerer, M.; Zaremba, S.; Pahr, D.H. Experimental investigation of a novel hybrid metal–composite joining technology. Compos. Part A Appl. Sci. Manuf. 2010, 41, 369–374. [Google Scholar] [CrossRef]
- Zhao, X.L.; Zhang, L. State-of-the-art review on FRP strengthened steel structures. Eng. Struct. 2007, 29, 1808–1823. [Google Scholar] [CrossRef]
- Cavaliere, F.; Aliaga, D. Part Made of a Composite, Including Lightning Protection Means. United. States Patent Application Publication No.: US 2012/0003495 A1, 5 January 2012. [Google Scholar]
- Brick, R.O.; Meyer, C.A. Lightning Protection for Electrically Conductive or Insulating Skin and Core for Honeycomb Structure. U.S. Patent 6,432,507 B1, 13 August 2002. [Google Scholar]
- Le Louarn, A.; Labal, F. System for Dissipating a Lightning Current Generated by a Thunderstorm Discharge on an Aircraft. U.S. Patent 8,699,203, 15 April 2014. [Google Scholar]
- Brown, A.M. Environmentally Stable Hybrid Fabric System for Exterior Protection of an Aircraft. U.S. Patent 8,524,620 B2, 3 September 2013. [Google Scholar]
- Le, Q.N.; Greegor, R.B. Method for Mitigating Edge Glow. United. States Patent Application Publication No. US 2016/0196891 A1, 7 July 2016. [Google Scholar]
- Drzal, L.T.; Rich, M.J.; Lloyd, P.F. Adhesion of graphite fibres to epoxy matrices: I. The role of fibre surface treatment. J. Adhes. 1983, 16, 1–30. [Google Scholar] [CrossRef]
- Shalin, R.E. (Ed.) Polymer Matrix Composites; Springer: Berlin/Heidelberg, Germany, 2012; Volume 4, pp. 1–2. [Google Scholar]
- Tuakta, C. Use of Fibre Reinforced Polymer Composite in Bridge Structures. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2005. [Google Scholar]
- Bernard Potyrala, P. Use of Fibre Reinforced Polymer Composites in Bridge Construction. State of the Art in Hybrid and All-Composite Structures. Master’s Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2011. [Google Scholar]
- Mangalgiri, P.D. Composite materials for aerospace applications. Bull. Mater. Sci. 1999, 22, 657–664. [Google Scholar] [CrossRef]
- Steinmann, W.; Saelhoff, A.K. Essential Properties of Fibres for Composite Applications. In Fibrous and Textile Materials for Composite Applications; Springer: Singapore, Singapore, 2016; pp. 39–73. [Google Scholar]
- Selzer, R.; Friedrich, K. Mechanical properties and failure behaviour of carbon fibre-reinforced polymer composites under the influence of moisture. Compos. Part A Appl. Sci. Manuf. 1997, 28, 595–604. [Google Scholar] [CrossRef]
- Bradley, J.S.; Hastings, G.W.; Johnson-Nurse, C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials 1980, 1, 38–40. [Google Scholar] [CrossRef]
- Brunbauer, J.; Stadler, H.; Pinter, G. Mechanical properties, fatigue damage and microstructure of carbon/epoxy laminates depending on fibre volume content. Int. J. Fatigue 2015, 70, 85–92. [Google Scholar] [CrossRef]
- Selvadurai, A.P.S.; Nikopour, H. Transverse elasticity of a unidirectionally reinforced composite with an irregular fibre arrangement: Experiments, theory and computations. Compos.Struct. 2012, 94, 1973–1981. [Google Scholar] [CrossRef]
- Harris, B.; Beaumont, P.W.R.; De Ferran, E.M. Strength and fracture toughness of carbon fibre polyester composites. J. Mater. Sci. 1971, 6, 238–251. [Google Scholar] [CrossRef]
- Park, J.K.; Kang, T.J. Thermal and ablative properties of low temperature carbon fibre–phenol formaldehyde resin composites. Carbon 2002, 40, 2125–2134. [Google Scholar] [CrossRef]
- Dhakate, S.R.; Bahl, O.P. Effect of carbon fibre surface functional groups on the mechanical properties of carbon–carbon composites with HTT. Carbon 2003, 41, 1193–1203. [Google Scholar] [CrossRef]
- Wonderly, C.; Grenestedt, J.; Fernlund, G.; Cěpus, E. Comparison of mechanical properties of glass fibre/vinyl ester and carbon fibre/vinyl ester composites. Compos. Part B Eng. 2005, 36, 417–426. [Google Scholar] [CrossRef]
- Marouani, S.; Curtil, L.; Hamelin, P. Ageing of carbon/epoxy and carbon/vinylester composites used in the reinforcement and/or the repair of civil engineering structures. Compos. Part B Eng. 2012, 43, 2020–2030. [Google Scholar] [CrossRef]
- Tekalur, S.A.; Shivakumar, K.; Shukla, A. Mechanical behavior and damage evolution in E-glass vinyl ester and carbon composites subjected to static and blast loads. Compos. Part B Eng. 2008, 39, 57–65. [Google Scholar] [CrossRef]
- Morgan, R.J.; Jurek, R.J.; Yen, A.; Donnellan, T. Toughening procedures, processing and performance of bismaleimide-carbon fibre composites. Polymer 1993, 34, 835–842. [Google Scholar] [CrossRef]
- Bao, L.R.; Yee, A.F. Effect of temperature on moisture absorption in a bismaleimide resin and its carbon fibre composites. Polymer 2002, 43, 3987–3997. [Google Scholar] [CrossRef]
- Bao, L.R.; Yee, A.F. Moisture diffusion and hygrothermal aging in bismaleimide matrix carbon fibre composites—Part I: Uni-weave composites. Compos. Sci. Technol. 2002, 62, 2099–2110. [Google Scholar] [CrossRef]
- Colin, X.; Marais, C.; Verdu, J. Kinetic modelling of the stabilizing effect of carbon fibres on thermal ageing of thermoset matrix composites. Compos. Sci. Technol. 2005, 65, 117–127. [Google Scholar] [CrossRef]
- Yudin, V.E.; Svetlichnyi, V.M.; Shumakov, A.N.; Schechter, R.; Harel, H.; Marom, G. Morphology and mechanical properties of carbon fibre reinforced composites based on semicrystalline polyimides modified by carbon nanofibres. Compos. Part A Appl. Sci. Manuf. 2008, 39, 85–90. [Google Scholar] [CrossRef]
- Xie, J.; Xin, D.; Cao, H.; Wang, C.; Zhao, Y.; Yao, L.; Ji, F.; Qiu, Y. Improving carbon fibre adhesion to polyimide with atmospheric pressure plasma treatment. Surf. Coat. Technol. 2011, 206, 191–201. [Google Scholar] [CrossRef]
- Nair, C.R.; Mathew, D.; Ninan, K.N. Cyanate ester resins, recent developments. In New Polymerization Techniques and Synthetic Methodologies; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–99. [Google Scholar]
- Chung, K.; Seferis, J.C. Evaluation of thermal degradation on carbon fibre/cyanate ester composites. Polym. Degrad. Stab. 2001, 71, 425–434. [Google Scholar] [CrossRef]
- Marieta, C.; Schulz, E.; Mondragon, I. Characterization of interfacial behaviour in carbon-fibre/cyanate composites. Compos. Sci. Technol. 2002, 62, 299–309. [Google Scholar] [CrossRef]
- Marieta, C.; Schulz, E.; Irusta, L.; Gabilondo, N.; Tercjak, A.; Mondragon, I. Evaluation of fibre surface treatment and toughening of thermoset matrix on the interfacial behaviour of carbon fibre-reinforced cyanate matrix composites. Compos. Sci. Technol. 2005, 65, 2189–2197. [Google Scholar] [CrossRef]
- Thunga, M.; Lio, W.Y.; Akinc, M.; Kessler, M.R. Adhesive repair of bismaleimide/carbon fibre composites with bisphenol E cyanate ester. Compos. Sci. Technol. 2011, 71, 239–245. [Google Scholar] [CrossRef]
- Bauer, A.; Thunga, M.; Obusek, K.; Akinc, M.; Kessler, M.R. Bisphenol E cyanate ester as a novel resin for repairing BMI/carbon fibre composites: Influence of cure temperature on adhesive bond strength. Polymer 2013, 54, 3994–4002. [Google Scholar] [CrossRef]
- Cogswell, F.N. Thermoplastic Aromatic Polymer Composites: A Study of the Structure, Processing and Properties of Carbon Fibre Reinforced Polyetheretherketone and Related Materials; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Wang, A.; Lin, R.; Polineni, V.K.; Essner, A.; Stark, C.; Dumbleton, J.H. Carbon fibre reinforced polyether ether ketone composite as a bearing surface for total hip replacement. Tribol. Int. 1998, 31, 661–667. [Google Scholar] [CrossRef]
- Saleem, A.; Frormann, L.; Iqbal, A. High performance thermoplastic composites: Study on the mechanical, thermal, and electrical resistivity properties of carbon fibre-reinforced polyetheretherketone and polyethersulphone. Polym. Compos. 2007, 28, 785–796. [Google Scholar] [CrossRef]
- Brockett, C.L.; John, G.; Williams, S.; Jin, Z.; Isaac, G.H.; Fisher, J. Wear of ceramic-on-carbon fibre-reinforced poly-ether ether ketone hip replacements. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, I.; Takao, M.; Goto, T.; Ohtsuki, C.; Hibino, S.; Sugano, N. Interfacial shear strength of bioactive-coated carbon fibre reinforced polyetheretherketone after in vivo implantation. J. Orthop. Res. 2012, 30, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Naffakh, M.; González-Domínguez, J.M.; Ansón, A.; Martínez-Rubi, Y.; Martinez, M.T.; Simard, B.; Gómez, M.A. High performance PEEK/carbon nanotube composites compatibilized with polysulfones-II. Mechanical and electrical properties. Carbon 2010, 48, 3500–3511. [Google Scholar] [CrossRef]
- Wenz, L.M.; Merritt, K.; Brown, S.A.; Moet, A.; Steffee, A.D. In vitro biocompatibility of polyetheretherketone and polysulfone composites. J. Biomed. Mater. Res. Part A 1990, 24, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.C.; Hastings, G.W.; Ohta, H.; Niwa, S.; Boeree, N. Development of a degradable composite for orthopaedic use: In vivo biomechanical and histological evaluation of two bioactive degradable composites based on the polyhydroxybutyrate polymer. Biomaterials 1992, 13, 491–496. [Google Scholar] [CrossRef]
- Latour, R.A.; Black, J. Development of FRP composite structural biomaterials: Fatigue strength of the fibre/matrix interfacial bond in simulated in vivo environments. J. Biomed. Mater. Res. Part A 1993, 27, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Latour, R.A.; Black, J. Development of FRP composite structural biomaterials: Ultimate strength of the fibre/matrix interfacial bond in vivo simulated environments. J. Biomed. Mater. Res. Part A 1992, 26, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Dickey, E.C.; Andrews, R.; Rantell, T. Load transfer and deformation mechanisms in carbon nanotube–polystyrene composites. Appl. Phys. Lett. 2000, 76, 2868–2870. [Google Scholar] [CrossRef]
- Patton, R.D.; Pittman, C.U.; Wang, L., Jr.; Hill, J.R. Vapor grown carbon fibre composites with epoxy and poly(phenylene sulfide) matrices. Compos. Part A Appl. Sci. Manuf. 1999, 30A, 1081–1091. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Q.; Wu, C.; Huang, J. Effect of carbon fibre reinforcement on the mechanical and tribological properties of polyamide6/polyphenylene sulfide composites. Mater. Des. 2013, 44, 493–499. [Google Scholar] [CrossRef]
- Stoeffler, K.; Andjelic, S.; Legros, N.; Roberge, J.; Schougaard, S.B. Polyphenylene sulfide (PPS) composites reinforced with recycled carbon fibre. Compos. Sci. Technol. 2013, 84, 65–71. [Google Scholar] [CrossRef]
- Carneiro, O.S.; Covas, J.A.; Bernardo, C.A.; Caldiera, G.; Van Hattum, F.W.J.; Ting, J.M. Production and assessment of polycarbonate composites reinforced with vapour-grown carbon fibres. Compos. Sci. Technol. 1998, 58, 401–407. [Google Scholar] [CrossRef]
- Paiva, M.C.; Nardin, M.; Bernardo, C.A.; Schultz, J. Influence of thermal history on the results of fragmentation tests on high-modulus carbon-fibre/polycarbonate model composites. Compos. Sci. Technol. 1997, 57, 839–843. [Google Scholar] [CrossRef]
- Hornbostel, B.; Pötschke, P.; Kotz, J.; Roth, S. Mechanical properties of triple composites of polycarbonate, single-walled carbon nanotubes and carbon fibres. Phys. ELow-Dimens. Syst. Nanostruct. 2008, 40, 2434–2439. [Google Scholar] [CrossRef]
- Kumar, S.; Doshi, H.; Srinivasarao, M.; Park, J.O.; Schiraldi, D.A. Fibres from polypropylne/nanocarbon fibre composites. Polymer 2002, 43, 1701–1703. [Google Scholar] [CrossRef]
- Varga, J.; Karger-Kocsis, J. Interfacial morphologies in carbon fibre-reinforced polypropylene microcomposites. Polymer 1995, 36, 4877–4881. [Google Scholar] [CrossRef]
- Rezaei, F.; Yunus, R.; Ibrahim, N.A.; Mahdi, E.D. Development of short-carbon-fibre-reinforced polypropylene composite for car bonnet. Polym. -Plast. Technol. Eng. 2008, 47, 351–357. [Google Scholar] [CrossRef]
- Rezaei, F.; Yunus, R.; Ibrahim, N.A. Effect of fibre length on thermomechanical properties of short carbon fibre reinforced polypropylene composites. Mater. Des. 2009, 30, 260–263. [Google Scholar] [CrossRef]
- Pogue, R.T.; Ye, J.; Klosterman, D.A.; Glass, A.S.; Chartoff, R.P. Evaluating fibre–matrix interaction in polymer–matrix composites by inverse gas chromatography. Compos. Part A 1998, 29A, 1273–1281. [Google Scholar] [CrossRef]
- Wang, J.; Gu, M.; Songhao, B.; Ge, S. Investigation of the influence of MoS2 filler on the tribological properties of carbon fibre reinforced nylon 1010 composites. Wear 2003, 255, 774–779. [Google Scholar] [CrossRef]
- Karsli, N.G.; Aytac, A. Tensile and thermomechanical properties of short carbon fibre reinforced polyamide 6 composites. Compos. Part B Eng. 2013, 51, 270–275. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.H. Friction and wear properties of surface-treated carbon fibre-reinforced thermoplastic polyimide composites under oil-lubricated condition. Mater. Chem. Phys. 2008, 108, 67–72. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Pei, X. Study on the synergistic effect of carbon fibre and graphite and nanoparticle on the friction and wear behavior of polyimide composites. Mater. Des. 2010, 31, 3761–3768. [Google Scholar] [CrossRef]
- Ogasawara, T.; Ishikawa, T.; Yokota, R.; Ozawa, H.; Taguchi, M.; Sato, R.; Shigenari, R.; Miyagawa, K. Processing and properties of carbon fibre reinforced triple-A polyimide (Tri-A PI) matrix composites. Adv. Compos. Mater. 2002, 11, 277–286. [Google Scholar] [CrossRef]
- Chellappa, V.; Chiou, Z.W.; Jang, B.Z. Electrical behavior of carbon whisker re inforced elastomer matrix composites. In Proceedings of the 26th SAMPE Technical Conference, Atlanta, GA, USA, 17–20 October 1994; pp. 12–18. [Google Scholar]
- Costa, P.; Silva, J.; Ansón-Casaos, A.; Martinez, M.T.; Abad, M.J.; Viana, J.; Lanceros-Mendez, S. Effect of carbon nanotube type and functionalization on the electrical, thermal, mechanical and electromechanical properties of carbon nanotube/styrene–butadiene–styrene composites for large strain sensor applications. Compos. Part B Eng. 2014, 61, 136–146. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.; Ribeiro, S.; Lanceros-Mendez, S. Mechanical vs. electrical hysteresis of carbon nanotube/styrene–butadiene–styrene composites and their influence in the electromechanical response. Compos. Sci. Technol. 2015, 109, 1–5. [Google Scholar] [CrossRef]
- Zhou, Y.; Pervin, F.; Jeelani, S.; Mallick, P.K. Improvement in mechanical properties of carbon fabric–epoxy composite using carbon nanofibres. J. Mater. Process. Technol. 2008, 198, 445–453. [Google Scholar] [CrossRef]
- Puglia, D.; Valentini, L.; Kenny, J.M. Analysis of the cure reaction of carbon nanotubes/epoxy resin composites through thermal analysis and Raman spectroscopy. J. Appl. Polym. Sci. 2003, 88, 452–458. [Google Scholar] [CrossRef]
- Fang, T.; Shimp, D.A. Polycyanate esters: Science and applications. Prog. Polym. Sci. 1995, 20, 61–118. [Google Scholar] [CrossRef]
- Hamerton, I.; Hay, J.N. Recent developments in the chemistry of cyanate esters. Polym. Int. 1998, 47, 465–473. [Google Scholar] [CrossRef]
- Hamerton, I.; Hay, J.N. Recent technological developments in cyanate ester resins. High Perform. Polym. 1998, 10, 163–174. [Google Scholar] [CrossRef]
- Reghunadhan Nair, C.P.; Francis, T.; Vijayan, T.M.; Krishnan, K. Sequential interpenetrating polymer networks from bisphenol A based cyanate ester and bismaleimide: Properties of the neat resin and composites. J. Appl. Polym. Sci. 1999, 74, 2737–2746. [Google Scholar] [CrossRef]
- Thomas, R.; Krishnamurthy, R. Effect of high-frequency low-amplitude impact loads on polymeric composites. J. Compos. Technol. Res. 2000, 22, 40–44. [Google Scholar]
- Herr, D.E.; Nikolic, N.A.; Schultz, R.A. Chemistries for high reliability in electronics assemblies. High Perform. Polym. 2001, 13, 79–100. [Google Scholar] [CrossRef]
- Hamerton, I. Properties of unreinforced cyanate ester matrix resins. In Chemistry and Technology of Cyanate Ester Resins; Hamerton, I., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 193–229. [Google Scholar]
- Njuguna, J.; Pielichowski, K. The role of advanced polymer materials in aerospace. Res.Gate 2013, 148. [Google Scholar]
- Guenthner, A.J.; Wright, M.E.; Chafin, A.P.; Reams, J.T.; Lamison, K.R.; Ford, M.D.; Kirby, S.P.; Zavala, J.J.; Mabry, J.M. Mechanisms of decreased moisture uptake in ortho-methylated di (cyanate ester) networks. Macromolecules 2014, 47, 7691–7700. [Google Scholar] [CrossRef]
- Chaplin, A.; Hamerton, I.; Herman, H.M.A.K.; Mudhar, A.K.; Shaw, S.J. Studying water uptake effects in resins based on cyanate ester/bismaleimide blends. Polymer 2000, 41, 3945–3956. [Google Scholar] [CrossRef]
- Ganguli, S.; Dean, D.; Jordan, K.; Price, G.; Vaia, R. Mechanical properties of intercalated cyanate ester–layered silicate nanocomposites. Polymer 2003, 44, 1315–1319. [Google Scholar] [CrossRef]
- Fan, J.; Hu, X.; Yue, C.Y. Dielectric properties of self-catalytic interpenetrating polymer network based on modified bismaleimide and cyanate ester resins. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1123–1134. [Google Scholar] [CrossRef]
- Schultz, J.; Lavielle, L. Interfacial Properties of Carbon Fibre—Epoxy Matrix Composites. In Inverse Gas Chromatography of ACS Symposium Series; ACS: Washington, DC, USA, 1989; Volume 391, Chapter 14; pp. 185–202. [Google Scholar]
- Kardos, J.L. The Role of the Interface in Polymer Composites — Some Myths, Mechanisms, and Modifications. In Polymer Science and Technology (27 Molecular Characterisation of Composite Interfaces); Ishida, H., Kumar, G., Eds.; Plenum: New York, NY, USA, 1985; pp. 1–11. [Google Scholar]
- Hughes, J.D.H. The Carbon Fibre/Epoxy Interface A Review. Compos. Sci. Technol. 1991, 41, 13–45. [Google Scholar] [CrossRef]
- Sharma, M.; Gao, S.; Mäder, E.; Sharma, H.; Wei, L.Y.; Bijwe, J. Carbon fibre surfaces and composite interphases. Compos. Sci. Technol. 2014, 102, 35–50. [Google Scholar] [CrossRef]
- Jones, C. The chemistry of carbon fibre surfaces and its effect on interfacial phenomena in fibre/epoxy composites. Compos. Sci. Technol. 1991, 42, 275–298. [Google Scholar] [CrossRef]
- Tiwari, S.; Bijwe, J. Surface treatment of carbon fibres—A review. Procedia Technol. 2014, 14, 505–512. [Google Scholar] [CrossRef]
- Donnet, J.B. Structure and reactivity of carbons: From carbon black to carbon composites. Carbon 1982, 20, 267–282. [Google Scholar] [CrossRef]
- Kozlowski, C.; Sherwood, P.M. X-ray photoelectron spectroscopic studies of carbon-fibre surfaces. Part 4—The effect of electrochemical treatment in nitric acid. J. Chem. Soc. Faraday Trans. 1 1984, 80, 2099–2107. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, C.; Zhang, S.; Lin, X. Effect of surface modification on carbon fibre and its reinforced phenolic matrix composite. Appl. Surf. Sci. 2012, 259, 288–293. [Google Scholar] [CrossRef]
- Yue, Z.R.; Jiang, W.; Wang, L.; Gardner, S.D.; Pittman, C.U. Surface characterization of electrochemically oxidized carbon fibres. Carbon 1999, 37, 1785–1796. [Google Scholar] [CrossRef]
- Gulyás, J.; Földes, E.; Lázár, A.; Pukánszky, B. Electrochemical oxidation of carbon fibres: Surface chemistry and adhesion. Compos. Part A Appl. Sci. Manuf. 2001, 32, 353–360. [Google Scholar] [CrossRef]
- Bian, X.S.; Ambrosio, L.; Kenny, J.M.; Nicolais, L.; Occhiello, E.; Morra, M.; Garbassi, F.; Dibenedetto, A.T. The effects of surface treatments of fibres on the interfacial properties in single-fibre composites. J. Adhes. Sci. Technol. 1991, 5, 377–388. [Google Scholar] [CrossRef]
- Dilsiz, N. Plasma surface modification of carbon fibres: A review. J. Adhes. Sci. Technol. 2000, 14, 975–987. [Google Scholar] [CrossRef]
- Kettle, A.P.; Beck, A.J.; O’toole, L.; Jones, F.R.; Short, R.D. Plasma polymerisation for molecular engineering of carbon-fibre surfaces for optimised composites. Compos. Sci. Technol. 1997, 57, 1023–1032. [Google Scholar] [CrossRef]
- Yamada, K.; Haraguchi, T.; Kajiyama, T. Plasma-graft polymerization of vinyl monomer with an acid amide group onto a surface of carbon fibre and its adhesion to epoxy resin. J. Appl. Polym. Sci. 2000, 75, 284–290. [Google Scholar] [CrossRef]
- Subramanian, R.V.; Jakubowski, J.J. Electropolymerization on graphite fibres. Polym. Eng. Sci. 1978, 18, 590–600. [Google Scholar] [CrossRef]
- Subramanian, R.V.; Sundaram, V.; Patel, A.K. Electrodeposition of polymers on graphite fibres: Effects on composite properties. In Proceedings of the 33rd Ann. Conf. Reinforced Plastics/Composites Int. Soc. Ind.; Section 20-F, Washington, DC, USA, February 1978; pp. 1–8. [Google Scholar]
- Xu, Z.; Huang, Y.; Zhang, C.; Chen, G. Influence of rare earth treatment on interfacial properties of carbon fibre/epoxy composites. Mater. Sci. Eng. A 2007, 444, 170–177. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhao, P.; Pei, X.Q.; Wang, Q.H.; Jia, Q. Flexural strength and tribological properties of rare earth treated short carbon fibre/polyimide composites. Expr. Polym. Lett. 2007, 1, 667–672. [Google Scholar] [CrossRef]
- Shangguan, Q.; Cheng, X. Effect of rare earths surface treatment on tribological properties of carbon fibres reinforced PTFE composite under oil-lubricated condition. J. Rare Earths 2008, 26, 584–589. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, W.Z.; Wang, D.Z.; Ren, Z.F.; Chou, T.W. Carbon nanotube/carbon fibre hybrid multiscale composites. J. Appl. Phys. 2002, 91, 6034–6037. [Google Scholar] [CrossRef]
- Zhang, F.H.; Wang, R.G.; He, X.D.; Wang, C.; Ren, L.N. Interfacial shearing strength and reinforcing mechanisms of an epoxy composite reinforced using a carbon nanotube/carbon fibre hybrid. J. Mater. Sci. 2009, 44, 3574–3577. [Google Scholar] [CrossRef]
- Wicks, S.S.; de Villoria, R.G.; Wardle, B.L. Interlaminar and intralaminar reinforcement of composite laminates with aligned carbon nanotubes. Compos. Sci. Technol. 2010, 70, 20–28. [Google Scholar] [CrossRef]
- Sharma, S.P.; Lakkad, S.C. Compressive strength of carbon nanotubes grown on carbon fibre reinforced epoxy matrix multi-scale hybrid composites. Surf. Coat. Technol. 2010, 205, 350–355. [Google Scholar] [CrossRef]
- Sharma, S.P.; Lakkad, S.C. Impact behavior and fractographic study of carbon nanotubes grafted carbon fibre-reinforced epoxy matrix multi-scale hybrid composites. Compos. Part A Appl. Sci. Manuf. 2015, 69, 124–131. [Google Scholar] [CrossRef]
- Qian, H.; Bismarck, A.; Greenhalgh, E.S.; Shaffer, M.S. Carbon nanotube grafted carbon fibres: A study of wetting and fibre fragmentation. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Gu, Y.; Liu, Y.; Li, Y.; Zhang, Z. Interfacial improvement of carbon fibre/epoxy composites using a simple process for depositing commercially functionalized carbon nanotubes on the fibres. Carbon 2013, 52, 109–121. [Google Scholar] [CrossRef]
- Hung, K.H.; Kuo, W.S.; Ko, T.H.; Tzeng, S.S.; Yan, C.F. Processing and tensile characterization of composites composed of carbon nanotube-grown carbon fibres. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1299–1304. [Google Scholar] [CrossRef]
- Fitzer, E.; Weiss, R. Effect of surface treatment and sizing of C-fibres on the mechanical properties of CFR thermosetting and thermoplastic polymers. Carbon 1987, 25, 455–467. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, M.K.; Rhee, K.Y. Effect of Ar+ ion beam irradiation on the physicochemical characteristics of carbon fibres. Carbon 2003, 41, 592–594. [Google Scholar] [CrossRef]
- Denison, P.; Jones, F.R.; Watts, J.F. The use of XPS and labelling techniques to study the surface chemistry of carbon fibres. J. Phys. D Appl. Phys. 1987, 20, 306. [Google Scholar] [CrossRef]
- Jacobasch, H.J.; Grundke, K.; Uhlmann, P.; Simon, F.; Mäder, E. Comparison of surface-chemical methods for characterizing carbon fibre-epoxy resin composites. Compos. Interfaces 1995, 3, 293–320. [Google Scholar] [CrossRef]
- Waltersson, K. ESCA studies of carbon fibres: Part III—Surface reactions of carbon fibres with amines. Compos. Sci. Technol. 1985, 23, 303–321. [Google Scholar] [CrossRef]
- Vickers, P.E.; Turner, M.E.; Abel, M.L.; Watts, J.F. The interaction of organic molecules with carbon fibre surfaces: A ToF-SIMS study. Compos. Part A Appl. Sci. Manuf. 1998, 29, 1291–1304. [Google Scholar] [CrossRef]
- Lindsay, B.; Abel, M.L.; Watts, J.F. A study of electrochemically treated PAN based carbon fibres by IGC and XPS. Carbon 2007, 45, 2433–2444. [Google Scholar] [CrossRef]
- Taylor, R.J.; Humffray, A.A. Electrochemical studies on glassy carbon electrodes: II. Oxygen reduction in solutions of high pH (pH > 10). J. Electroanal. Chem. Interfacial Electrochem. 1975, 64, 63–84. [Google Scholar] [CrossRef]
- Sloan, F.E.; Talbot, J.B. Corrosion of graphite-fibre-reinforced composites I-galvanic coupling damage. Corrosion 1992, 48, 830–838. [Google Scholar] [CrossRef]
- Alias, M.N.; Brown, R. Damage to composites from electrochemical processes. Corrosion 1992, 48, 373–378. [Google Scholar] [CrossRef]
- Kaushik, D.; Alias, M.N.; Brown, R. An impedance study of a carbon fibre/vinyl ester composite. Corrosion 1991, 47, 859–867. [Google Scholar] [CrossRef]
- Woo, E.M.; Chen, J.S.; Carter, C.S. Mechanisms of degradation of polymer composites by galvanic reactions between metals and carbon fibre. Polym. Compos. 1993, 14, 395–401. [Google Scholar] [CrossRef]
- Hine, P.J.; Brew, B.; Duckett, R.A.; Ward, I.M. The fracture behaviour of carbon fibre reinforced poly(ether etherketone). Compos. Sci. Technol. 1988, 33, 35–71. [Google Scholar] [CrossRef]
- Hine, P.J.; Brew, B.; Duckett, R.A.; Ward, I.M. Failure mechanisms in continuous carbon fibre reinforced PEEK composites. Compos. Sci. Technol. 1989, 35, 31–51. [Google Scholar] [CrossRef]
- Hine, P.J.; Brew, B.; Duckett, R.A.; Ward, I.M. Failure mechanisms in carbon fibre reinforced poly(etheretherketone), II: Material variables. Compos. Sci. Technol. 1991, 40, 47–67. [Google Scholar] [CrossRef]
- Morgan, P. Carbon Fibres and Their Composites; CRC Press: Boca Raton, FL, USA, 2005; pp. 540–543. [Google Scholar]
- Johnston, N.J. (Ed.) Toughened Composites ASTM STP937; American Society for Testing and Materials: Philidelphia, PA, USA, 1987. [Google Scholar]
- Pipes, R.B.; Pagano, N.J. Interlaminar stresses in composite laminates under uniform axial extension. J. Compos. Mater. 1970, 4, 538–548. [Google Scholar] [CrossRef]
- Puppo, A.H.; Evensen, H.A. Interlaminar shear in laminated composites under generalized plane stress. J. Compos. Mater. 1970, 4, 204–220. [Google Scholar] [CrossRef]
- Purslow, D. Some fundamental aspects of composites fractography. Composites 1981, 12, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chung, D.D.L. Interlaminar shear in carbon fibre polymer-matrix composites, studied by measuring the contact electrical resistance of the interlaminar interface during shear. Compos. Interfaces 1998, 6, 507–517. [Google Scholar] [CrossRef]
- Naik, N.K.; Asmelash, A.; Kavala, V.R.; Ch, V. Interlaminar shear properties of polymer matrix composites: Strain rate effect. Mech. Mater. 2007, 39, 1043–1052. [Google Scholar] [CrossRef]
- Drazal, L.; Madhukar, M. Fibre-matrix adhesion and its relationship to composite mechanical properties. J. Mater. Sci. 1993, 28, 569–610. [Google Scholar] [CrossRef]
- ISO 1413 ISO 14130:1997—Fibre-Reinforced Plastic Composites—Determination of Apparent Interlaminar Shear Strength by Short-Beam Method; International Organization for Standardization: Geneva, Switzerland, 1997.
- Saelhoff, A.K.; Jäger, M.; Steinmann, W.; Gries, T. Surface treatment of carbon fibres—Increasing the interlaminar shear strength in CFRP. In Proceedings of the ADITC2014 Aachen-Dresdner International Textile Conference, Dresden, Germany, 27–28 November 2014. [Google Scholar]
- Steinmann, W.; Wulfhorst, J.; Walter, S.; Seide, G.; Gries, T. Nanoparticles in polymeric fibres: Novel possibilities for the modification of surface, mechanical and electrical properties. In Proceedings of the Nanoscience Conference, Yaiza, Spain, 14–17 February 2012. [Google Scholar]
- Gojny, F.H.; Wichmann, M.H.G.; Fiedler, B.; Bauhofer, W.; Schulte, K. Influence of nano-modification on the mechanical and electrical properties of conventional fibre-reinforced composites. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1525–1535. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.; Fiedler, B.; Schulte, K. Influence of different carbon nanotubes on the mechanical properties of epoxy matrix composites—A comparative study. Compos. Sci. Technol. 2005, 65, 2300–2313. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, C.; Wang, B.; Liang, R. Carbon nanotube integrated multifunctional multiscale composites. Nanotechnology 2007, 18, 275708. [Google Scholar] [CrossRef]
- Lavine, M. The right combination. Science 2006, 314, 1099. [Google Scholar] [CrossRef]
- Reynaud, E.; Gauthier, C.; Perez, J. Nanophases in polymers. Rev. Metall. 1999, 96, 169–176. [Google Scholar] [CrossRef]
- Bekyarova, E.; Thostenson, E.T.; Yu, A.; Itkis, M.E.; Fakhrutdinov, D.; Chou, T.W.; Haddon, R.C. Functionalized single-walled carbon nanotubes for carbon fibre−epoxy composites. J. Phys. Chem. C 2007, 111, 17865–17871. [Google Scholar] [CrossRef]
- Bekyarova, E.; Thostenson, E.T.; Yu, A.; Kim, H.; Gao, J.; Tang, J.; Hahn, H.T.; Chou, T.W.; Itkis, M.E.; Haddon, R.C. Multiscale carbon nanotube−carbon fibre reinforcement for advanced epoxy composites. Langmuir 2007, 23, 3970–3974. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, Y.; Ishiwata, S.; Sumizawa, T.; Ishikawa, T. Mechanical properties improvements in two-phase and three-phase composites using carbon nano-fibre dispersed resin. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1430–1439. [Google Scholar] [CrossRef]
- Chou, T.W.; Gao, L.; Thostenson, E.T.; Zhang, Z.; Byun, J.H. An assessment of the science and technology of carbon nanotube-based fibres and composites. Compos. Sci. Technol. 2010, 70, 1–19. [Google Scholar] [CrossRef]
- Bal, S. Experimental study of mechanical and electrical properties of carbon nanofibre/epoxy composites. Mater. Des. 2010, 31, 2406–2413. [Google Scholar] [CrossRef]
- Green, K.J.; Dean, D.R.; Vaidya, U.K.; Nyairo, E. Multiscale fibre reinforced composites based on a carbon nanofibre/epoxy nanophased polymer matrix: Synthesis, mechanical, and thermomechanical behavior. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1470–1475. [Google Scholar] [CrossRef]
- García, E.J.; Hart, A.J.; Wardle, B.L. Long carbon nanotubes grown on the surface of fibres for hybrid composites. AIAA J. 2008, 46, 1405. [Google Scholar] [CrossRef]
- Wardle, B.L. Nanocomposites and nano-engineered composites reinforced with aligned carbon nanotubes. In Proceedings of the 17th International Conference on Composite Materials, Edinburgh, UK, 27–31 July 2009. [Google Scholar]
- Imanaka, M.; Nakamura, Y.; Nishimura, A.; Iida, T. Fracture toughness of rubber-modified epoxy adhesives: Effect of plastic deformability of the matrix phase. Compos. Sci. Technol. 2003, 63, 41–51. [Google Scholar] [CrossRef]
- Chikhi, N.; Fellahi, S.; Bakar, M. Modification of epoxy resin using reactive liquid (ATBN) rubber. Eur. Polym. J. 2002, 38, 251–264. [Google Scholar] [CrossRef]
- Xian, G.J.; Walter, R.; Haupert, F. Friction and wear of epoxy/TiO2 nanocomposites: Influence of additional short carbon fibres, Aramid and PTFE particles. Compos. Sci. Technol. 2006, 66, 3199–3209. [Google Scholar] [CrossRef]
- Vasconcelos, P.V.; Lino, F.J.; Magalhaes, A.; Neto, R.J.L. Impact fracture study of epoxy-based composites with aluminium particles and milled fibres. J. Mater. Process. Technol. 2005, 170, 277–283. [Google Scholar] [CrossRef]
- Zhou, Y.; Pervin, F.; Rangari, V.K.; Jeelani, S. Fabrication and evaluation of carbon nano fibre filled carbon/epoxy composite. Mater. Sci. Eng. A 2006, 426, 221–228. [Google Scholar] [CrossRef]
- Zhou, Y.; Pervin, F.; Lewis, L.; Jeelani, S. Fabrication and characterization of carbon/epoxy composites mixed with multi-walled carbon nanotubes. Mater. Sci. Eng. A 2008, 475, 157–165. [Google Scholar] [CrossRef]
- Sandler, J.; Shaffer, M.S.P.; Prasse, T.; Bauhofe, W.; Schulte, K.; Windle, A.H. Development of a dispersion process for carbon nanotubes in an epoxy matrix and the resulting electrical properties. Polymer 1999, 40, 5967–5971. [Google Scholar] [CrossRef]
- Lee, H.; Mall, S.; He, P.; Shi, D.L.; Narasimhadevara, S.; Yeo-Heung, Y.; Shanov, V.; Schulz, M. Characterization of carbon nanotube/nanofibre-reinforced polymer composites using an instrumented indentation technique. Compos. Part B Eng. 2007, 38, 58–65. [Google Scholar] [CrossRef]
- Qian, H.; Bismarck, A.; Greenhalgh, E.S.; Kalinka, G.; Shaffer, M.S. Hierarchical composites reinforced with carbon nanotube grafted fibres: The potential assessed at the single fibre level. Chem. Mater. 2008, 20, 1862–1869. [Google Scholar] [CrossRef]
- Mei, L.; Li, Y.; Wang, R.; Wang, C.; Peng, Q.; He, X. Multiscale carbon nanotube-carbon fibre reinforcement for advanced epoxy composites with high interfacial strength. Polym. Polym. Compos. 2011, 19, 107. [Google Scholar]
- Veedu, V.P.; Cao, A.; Li, X.; Ma, K.; Soldano, C.; Kar, S.; Ajayan, P.M.; Ghasemi-Nejhad, M.N. Multifunctional composites using reinforced laminae with carbon-nanotube forests. Nat. Mater. 2006, 5, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Sager, R.J.; Klein, P.J.; Lagoudas, D.C.; Zhang, Q.; Liu, J.; Dai, L.; Baur, J.W. Effect of carbon nanotubes on the interfacial shear strength of T650 carbon fibre in an epoxy matrix. Compos. Sci. Technol. 2009, 69, 898–904. [Google Scholar] [CrossRef]
- Mathur, R.B.; Chatterjee, S.; Singh, B.P. Growth of carbon nanotubes on carbon fibre substrates to produce hybrid/phenolic composites with improved mechanical properties. Compos. Sci. Technol. 2008, 68, 1608–1615. [Google Scholar] [CrossRef]
- Maruyama, B.; Alam, K. Carbon nanotubes and nanofibres in composite materials. SAMPE J. 2002, 38, 59–70. [Google Scholar]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-assisted highly efficient synthesis of impurity-free singlewalled carbon nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.W.; Sheehan, P.E.; Lieber, C.M. Nanobeam mechanics: Elasticity, strength, and toughness of nanorods and nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Yu, M.; Lourie, O.; Dyer, M.J.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, W.; Pan, Z.; Chang, B.; Sun, L. Mechanical and physical properties on carbon nanotube. J. Phys. Chem. Solids 2000, 61, 1153–1158. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.H.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Sugimoto, K.; Song, S.; Gotoh, Y.; Ohkoshi, Y.; Endo, M. Mechanical and physical properties of epoxy composites reinforced by vapor grown carbon nanofibres. Carbon 2005, 43, 2199–2208. [Google Scholar] [CrossRef]
- Gibson, T.; Rice, B.; Ragland, W.; Silverman, E.M.; Peng, H.; Strong, K.L.; Moon, D. Formulation and evaluation of carbon nanofibre-based conductive adhesives. In Proceedings of the SAMPE 2005, Long Beach, CA, USA, 1–5 May 2005. [Google Scholar]
- Kotaki, M.; Wang, K.; Toh, M.L.; Chen, L.; Wong, S.Y.; He, C. Electrically conductive epoxy/clay/vapor grown carbon fibre hybrids. Macromolecules 2006, 39, 908–911. [Google Scholar] [CrossRef]
- Fukushima, H.; Drzal, L.T. Graphite nanoplatelets as reinforcements for polymers: Structural and electrical properties. In Proceedings of the American Society for Composites 2002, 17th Technical Conference, West Lafayette, IN, USA, 21–23 October 2002. [Google Scholar]
- Lafdi, K. Effect of functionalization on properties of polymeric nanocomposites. In Proceedings of the VGCF Materials and Application Workshop 2004, Kettering, OH, USA, 14–15 September 2004. [Google Scholar]
- Allaoui, A.; Hoa, S.V.; Pugh, M.D. Effect of the preparation method on the electrical properties of carbon nanofibre/epoxy nanocomposites. In Proceedings of the Sixth Joint Canada–Japan Workshop on Composites 2006, Toronto, ON, Canada, 24–26 August 2006; pp. 43–51. [Google Scholar]
- Tibbetts, G.G.; Max, M.L.; Lake, L.; Strong, K.L.; Rice, B.P. A review of the fabrication and properties of vapor-grown carbon nanofibre/polymer composites. Compos. Sci. Technol. 2007, 67, 1709–1718. [Google Scholar] [CrossRef]
- Steinmann, W.; Vad, T.; Weise, B.; Wulfhorst, J.; Seide, G.; Gries, T.; Heidelmann, M.; Weirich, T. Extrusion of CNT-modified polymers with low viscosity—Influence of crystallization and CNT orientation on the electrical properties. Polym. Polym. Compos. 2013, 21, 473–482. [Google Scholar] [CrossRef]
- Cipriano, B.H.; Kota, A.K.; Gershon, A.L.; Laskowski, C.J.; Kashiwagi, T.; Bruck, H.A.; Raghavan, S.R. Conductivity enhancement of carbon nanotube and nanofibre-based polymer nanocomposites by melt annealing. Polymer 2008, 49, 4846–4851. [Google Scholar] [CrossRef]
- Lozano, K.; Bonilla-Rios, J.; Barrera, E.V. A study on nanofibre-reinforced thermoplastic composites (II): Investigation of the mixing rheology and conduction properties. J. Appl. Polym. Sci. 2001, 80, 1162–1172. [Google Scholar] [CrossRef]
- Pötschke, P.; Abdel-Goad, M.; Alig, I.; Dudkin, S.; Lellinger, D. Rheological and dielectrical characterization of melt mixed polycarbonate-multiwalled carbon nanotube composites. Polymer 2004, 45, 8863–8870. [Google Scholar] [CrossRef]
- Sandler, J.K.W.; Kirk, J.E.; Kinloch, I.A.; Shaffer, M.S.P.; Windle, A.H. Ultralow electrical percolation threshold in carbon-nanotube-epoxy composites. Polymer 2003, 44, 5893–5899. [Google Scholar] [CrossRef]
- Alig, I.; Skipa, T.; Engel, M.; Lellinger, D.; Pegel, S.; Pötschke, P. Electrical conductivity recovery in carbon nanotube–polymer composites after transient shear. Phys. Status Solidi 2007, 244, 4223–4226. [Google Scholar] [CrossRef]
- Pötschke, P.; Bhattacharyya, A.R.; Alig, I.; Dudkin, S.M.; Leonhardt, A.; Täschner, C.; Ritschel, M.; Roth, S.; Hornbostel, B.; Cech, J. Dispersion of carbon nanotubes into thermoplastic polymers using melt mixing. AIP Conf. Proc. 2004, 723, 478–484. [Google Scholar]
- Pötschke, P.; Dudkin, S.M.; Alig, I. Dielectric spectroscopy on melt processed polycarbonate—Multiwalled carbon nanotube composites. Polymer 2003, 44, 5023–5030. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Nesheva, D.; Nedkov, I.; Krusteva, E.; Stavrev, S. Reological, electrical and microwave properties of polymers with nanosized carbon particles. J. Appl. Polym. Sci. 2004, 92, 2220–2227. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 9, p. 9. [Google Scholar]
- Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAuA). Vermeidung von Zündgefahren infolge elektrostatischer Aufladungen; BAuA: Dortmund, Germany, 2009. [Google Scholar]
- ANSI/ESD S541-2008. For the Protection of Electrostatic Discharge Susceptible Items: Packaging Materials for ESD Sensitive Items; Electrostatic Discharge Association: Rome, NY, USA, 2008. [Google Scholar]
- Ireland, R.; Arronche, L.; La Saponara, V. Electrochemical investigation of galvanic corrosion between aluminum 7075 and glass fiber/epoxy composites modified with carbon nanotubes. Compos. Part B Eng. 2012, 43, 183–194. [Google Scholar] [CrossRef]
- Baltzis, D.; Orfanidis, S.; Lekatou, A.; Paipetis, A.S. Stainless steel coupled with carbon nanotube-modified epoxy and carbon fibre composites: Electrochemical and mechanical study. Plast. Rubber Compos. 2016, 45, 95–105. [Google Scholar] [CrossRef]
- Arronche, L.; Gordon, K.; Ryu, D.; La Saponara, V.; Cheng, L. Investigation of galvanic corrosion between AISI 1018 carbon steel and CFRPs modified with multi-walled carbon nanotubes. J. Mater. Sci. 2013, 48, 1315–1323. [Google Scholar] [CrossRef]
- Ofoegbu, S.U. Corrosion and Corrosion Inhibition in Multi-material Combinations. Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, 2018. [Google Scholar]
- Morcos, I.; Yeager, E. Kinetic studies of the oxygen—Peroxide couple on pyrolytic graphite. Electrochim. Acta 1970, 15, 953–975. [Google Scholar] [CrossRef]
- Yeager, E. Electrocatalysts for O2 reduction. Electrochim. Acta 1984, 29, 1527–1537. [Google Scholar] [CrossRef]
- Fischer, P.; Heitbaum, J. Mechanistic aspects of cathodic oxygen reduction. J. Electroanal. Chem. Interfacial Electrochem. 1980, 112, 231–238. [Google Scholar] [CrossRef]
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties; John Wiley and Sons: New York, NY, USA, 1988; pp. 360–372. [Google Scholar]
- Maldonado, S.; Stevenson, K.J. Influence of nitrogen doping on oxygen reduction electrocatalysis at carbon nanofibre electrodes. J. Phys. Chem. B 2005, 109, 4707–4716. [Google Scholar] [CrossRef] [PubMed]
- Mrha, J. Study of catalysts for fuel cell electrodes. IV. Active carbon electrodes for oxygen in alkaline electrolyte. Collect. Czechoslov. Chem. Commun. 1967, 32, 708–719. [Google Scholar] [CrossRef]
- Xu, J.; Huang, W.; McCreery, R.L. Isotope and surface preparation effects on alkaline dioxygen reduction at carbon electrodes. J. Electroanal. Chem. 1996, 410, 235–242. [Google Scholar] [CrossRef]
- Yang, H.H.; McCreery, R.L. Elucidation of the Mechanism of Dioxygen Reduction on Metal-Free Carbon Electrodes. J. Electrochem. Soc. 2000, 147, 3420–3428. [Google Scholar] [CrossRef]
- Taylor, R.J.; Humffray, A.A. Electrochemical studies on glassy carbon electrodes: III. Oxygen reduction in solutions of low pH (pH <10). J. Electroanal. Chem. Interfacial Electrochem. 1975, 64, 85–94. [Google Scholar]
- Boehm, H.P.; Diehl, E.; Heck, W.; Sappok, R. Surface oxides of carbon. Angew. Chem. 1964, 3, 669–677. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Lopez-Ramon, M.V.; Carrasco-Marın, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Bismarck, A.; Wuertz, C.; Springer, J. Basic surface oxides on carbon fibres. Carbon 1999, 37, 1019–1027. [Google Scholar] [CrossRef]
- Barton, S.S.; Boulton, G.L.; Harrison, B.H. Surface studies on graphite: Acidic surface oxides. Carbon 1972, 10, 395–400. [Google Scholar] [CrossRef]
- Dastgheib, S.A.; Karanfil, T.; Cheng, W. Tailoring activated carbons for enhanced removal of natural organic matter from natural waters. Carbon 2004, 42, 547–557. [Google Scholar] [CrossRef]
- Evans, M.J.B.; Halliop, E.; MacDonald, J.A.F. The production of chemically-activated carbon. Carbon 1999, 37, 269–274. [Google Scholar] [CrossRef]
- Shen, W.; Li, Z.; Liu, Y. Surface chemical functional groups modification of porous carbon. Recent Patents Chem. Eng. 2008, 1, 27–40. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfao, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Strelko, V.; Malik, D.J.; Streat, M. Characterisation of the surface of oxidised carbon adsorbents. Carbon 2002, 40, 95–104. [Google Scholar] [CrossRef]
- Jannakoudakis, A.D.; Jannakoudakis, P.D.; Theodoridou, E.; Besenhard, J.O. Electrochemical oxidation of carbon fibres in aqueous solutions and analysis of the surface oxides. J. Appl. Electrochem. 1990, 20, 619–624. [Google Scholar] [CrossRef]
- Boehm, H.P. Functional groups on the surfaces of solids. Angew. Chem. 1966, 5, 533–544. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Fritz, H.P. The electrochemistry of black carbons. Angew. Chem. 1983, 22, 950–975. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Jakob, J.; Krebber, U.; Moeller, P.; Sauter, R.F.; Kurtze, A.; Kanani, N.; Meyer, H.; Hoerber, J.K.H.; Jannakoudakis, A.D. Anodische Oberflächen-und Volumenoxidation graphitischer Materialien in neutralen und alkalischen wäßrigen Lösungen/Anodic Surface and Bulk Oxidation of Graphitic Materials in Neutral and Basic Aqueous Solutions. Zeitschrift für Naturforschung B 1989, 44, 729–735. [Google Scholar] [CrossRef]
- Zuleta, M.; Björnbom, P.; Lundblad, A. Characterization of the electrochemical and ion-transport properties of a nanoporous carbon at negative polarization by the single-particle method. J. Electrochemical Soc. 2006, 153, A48–A57. [Google Scholar] [CrossRef]
- Ruiz, V.; Blanco, C.; Raymundo-Piñero, E.; Khomenko, V.; Béguin, F.; Santamaría, R. Effects of thermal treatment of activated carbon on the electrochemical behaviour in supercapacitors. Electrochim. Acta 2007, 52, 4969–4973. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, V.; Malmberg, H.; Blanco, C.; Lundblad, A.; Björnbom, P.; Santamaría, R. A study of Faradaic phenomena in activated carbon by means of macroelectrodes and single particle electrodes. J. Electroanal. Chem. 2008, 618, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Béguin, F.; Kierzek, K.; Friebe, M.; Jankowska, A.; Machnikowski, J.; Jurewicz, K.; Frackowiak, E. Effect of various porous nanotextures on the reversible electrochemical sorption of hydrogen in activated carbons. Electrochim. Acta 2006, 51, 2161–2167. [Google Scholar] [CrossRef]
- Basova, Y.V.; Hatori, H.; Yamada, Y.; Miyashita, K. Effect of oxidation–reduction surface treatment on the electrochemical behavior of PAN-based carbon fibres. Electrochem. Commun. 1999, 1, 540–544. [Google Scholar] [CrossRef]
- Yeager, E.J.; Molla, J.A.; Gupta, S. The Electrochemistry of Carbon; Sarangapani, S., Akridge, J.R., Schumm, B., Eds.; PV 84-5; The Electrochemical Society Softbound Proceedings Series; The Electrochemical Society: Pennington, NJ, USA, 1984; p. 123. [Google Scholar]
- Kalnin, I.L. Preprints 2nd Int. Carbon Conf, Deutsche Keramische Gesellschaft, Bad Honnef, FRG; The Electrochemical Society: Pennington, NJ, USA, 1976; pp. 94–98. [Google Scholar]
- Kalnin, I. Extended abstracts - 14th Biennial Conference on Carbon; The Pennsylvania State University: University Park, PA, USA, 1979 25–29 June. [Google Scholar]
- Weinberg, N. The Electrochemistry of Carbon; Sarangapani, S., Akridge, J.R., Schumm, B., Eds.; PV 84-5; The Electrochemical Society Softbound Proceedings Series; The Electrochemical Society: Pennington, NJ, USA, 1984; p. 463. [Google Scholar]
- Allred, C.D.; McCreery, R.L. Adsorption of catechols on fractured glassy carbon electrode surfaces. Anal. Chem. 1992, 64, 444–448. [Google Scholar] [CrossRef]
- Chen, P.; McCreery, R.L. Control of electron transfer kinetics at glassy carbon electrodes by specific surface modification. Anal. Chem. 1996, 68, 3958–3965. [Google Scholar] [CrossRef]
- Chen, P.; Fryling, M.A.; McCreery, R.L. Electron transfer kinetics at modified carbon electrode surfaces: The role of specific surface sites. Anal. Chem. 1995, 67, 3115–3122. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Weiss, D.J.; Chong, S.H.; Kuwana, T. Charge-selective electrochemistry at high-surface-area carbon fibres. Anal. Chem. 1999, 71, 413–418. [Google Scholar] [CrossRef]
- Kelly, R.S.; Coleman, B.D.; Huang, T.; Inkaew, P.; Kuwana, T. Electrochemical flow injection analysis study of ion partitioning at high surface area carbon fibre electrodes. Anal. Chem. 2002, 74, 6364–6369. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.J.; Kelly, R.S.; Cumaranatunge, M.; Kuwana, T. Computer simulation of charge-selective electrochemistry of catechols at high-surface-area carbon fibres. Anal. Chem. 1999, 71, 3712–3720. [Google Scholar] [CrossRef] [PubMed]
- Gotch, A.J.; Kelly, R.S.; Kuwana, T. Characterization and modeling of the nonfaradaic response of ultrahigh surface area carbon fibres by electrochemical flow injection analysis. J. Phys. Chem. B 2003, 107, 935–941. [Google Scholar] [CrossRef]
- Kinoshita, K.; Chu, X. Carbon for supercapacitors. In Proceedings of the Symposium on Electrochemical Capacitors; Delnick, F., Tomkiewicz, M., Eds.; The Electrochemical Society: Pennington, NJ, USA, 1996; Volume 95–29, p. 171. [Google Scholar]
- Chu, X.; Kinoshita, K. Surface modification of carbons for enhanced electrochemical activity. Mater. Sci. Eng. B 1997, 49, 53–60. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, Y.; Li, Y.; Li, C.; Peng, H.; Zhang, J.; Liu, Z.; Dai, L.; Shi, G. The edge-and basal-plane-specific electrochemistry of a single-layer graphene sheet. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Shen, A.; Zou, Y.; Wang, Q.; Dryfe, R.A.; Huang, X.; Dou, S.; Dai, L.; Wang, S. Oxygen reduction reaction in a droplet on graphite: Direct evidence that the edge is more active than the basal plane. Angew. Chem. 2014, 126, 10980–10984. [Google Scholar] [CrossRef]
- Ji, X.; Banks, C.E.; Xi, W.; Wilkins, S.J.; Compton, R.G. Edge plane sites on highly ordered pyrolytic graphite as templates for making palladium nanowires via electrochemical decoration. J. Phys. Chem. B 2006, 110, 22306–22309. [Google Scholar] [CrossRef]
- Randin, J.P.; Yeager, E. Differential capacitance study of stress-annealed pyrolytic graphite electrodes. J. Electrochem. Soc. 1971, 118, 711–714. [Google Scholar] [CrossRef]
- Rao, C.V.; Cabrera, C.R.; Ishikawa, Y. In search of the active site in nitrogen-doped carbon nanotube electrodes for the oxygen reduction reaction. J. Phys. Chem. Lett. 2010, 1, 2622–2627. [Google Scholar] [CrossRef]
- Buan, M.E.; Muthuswamy, N.; Walmsley, J.C.; Chen, D.; Rønning, M. Nitrogen-doped carbon nanofibers on expanded graphite as oxygen reduction electrocatalysts. Carbon 2016, 2016, 191–202. [Google Scholar] [CrossRef]
- Wiggins-Camacho, J.D.; Stevenson, K.J. Mechanistic discussion of the oxygen reduction reaction at nitrogen-doped carbon nanotubes. J. Phys. Chem. C 2011, 115, 20002–20010. [Google Scholar] [CrossRef]
- Maldonado, S.; Stevenson, K.J. Direct preparation of carbon nanofibre electrodes via pyrolysis of iron (II) phthalocyanine: Electrocatalytic aspects for oxygen reduction. J. Phys. Chem. B 2004, 108, 11375–11383. [Google Scholar] [CrossRef]
- Vijayaraghavan, G.; Stevenson, K.J. Synergistic assembly of dendrimer-templated platinum catalysts on nitrogen-doped carbon nanotube electrodes for oxygen reduction. Langmuir 2007, 23, 5279–5282. [Google Scholar] [CrossRef]
- Sinmazçelik, T.; Avcu, E.; Bora, M.Ö.; Çoban, O. A review: Fibre metal laminates, background, bonding types and applied test methods. Mater. Des. 2011, 32, 3671–3685. [Google Scholar] [CrossRef]
- Nagatsuka, K.; Xiao, B.; Wu, L.; Nakata, K.; Saeki, S.; Kitamoto, Y.; Iwamoto, Y. Resistance spot welding of metal/carbon-fibre-reinforced plastics and applying silane coupling treatment. Sci. Technol. Weld. Joining 2018, 23, 181–186. [Google Scholar] [CrossRef]
- Balle, F.; Wagner, G.; Eifler, D. Ultrasonic metal welding of aluminium sheets to carbon fibre reinforced thermoplastic composites. Adv. Eng. Mater. 2009, 11, 35–39. [Google Scholar] [CrossRef]
- Amancio-Filho, S.T.; Bueno, C.; Dos Santos, J.F.; Huber, N.; Hage, E. On the feasibility of friction spot joining in magnesium/fibre-reinforced polymer composite hybrid structures. Mater. Sci. Eng. A 2011, 528, 3841–3848. [Google Scholar] [CrossRef]
- Jung, K.W.; Kawahito, Y.; Takahashi, M.; Katayama, S. Laser direct joining of carbon fibre reinforced plastic to zinc-coated steel. Mater. Des. 2013, 47, 179–188. [Google Scholar] [CrossRef]
- Uvarov, N.F.; Mateyshina, Y.G.; Ulihin, A.S.; Yusin, S.I.; Varentsova, V.I.; Varentsov, V.K. Surface Electrochemical Treatment of Carbon Materials for Supercapacitors. ECS Trans. 2010, 25, 11–16. [Google Scholar]
- Tucker, W.C.; Brown, R.; Russell, L. Corrosion between a graphite/polymer composite and metals. J. Compos. Mater. 1990, 24, 92–102. [Google Scholar] [CrossRef]
- Sloan, F.E. Chemical attack of graphite/epoxy by hydrogen peroxide. Appl. Spectrosc. 1992, 46, 524–528. [Google Scholar] [CrossRef]
- Schirmann, J.P.; Delavarenne, S.Y. Hydrogen Peroxide in Organic Chemistry; Edition et Documentation Industrielle: Paris, France, 1979. [Google Scholar]
- Sloan, F.E.; Talbot, J.B. Evolution of perhydroxyl ions on graphite/epoxy cathodes. J. Electrochem. Soc. 1997, 144, 4146–4151. [Google Scholar] [CrossRef]
- Pauly, C.C.; Taylor, S.R.; Gomez, J.P. Final Report Environmental Durability of Graphite/Epoxy Composites: The Combined Effects of Moisture, Cathodic Polarization, and Stress; No. FHWA/VTRC 02-R13; Virginia Transportation Research Council: Charlottesville, WV, USA, 2002.
- Taylor, S.R.; Cahen, G.L., Jr. The Detection and Analysis of Galvanic Damage in BMI/Graphite Fibre Composites. In Proceedings of the Corrosion Detection and Management of Advanced Airframe Materials; Agard Conference Proceedings 565, 79th Meeting of the AGARD Structures and Materials Panel, Seville, Spain, 5–6 October 1994; pp. 6-1–6-12. [Google Scholar]
- Taylor, S.R.; Wall, F.D.; Cahen, G.L. The detection and analysis of electrochemical damage in bismaleimide/graphite fibre composites. J. Electrochem. Soc. 1996, 143, 449–458. [Google Scholar] [CrossRef]
- Kawai, F. Biodegradable Polymers and Plastics; Vert, M., Feijen, J., Albertsson, A., Scott, G., Chiellini, F., Eds.; The Royal Society of Chemistry, Redwood Press: Wiltshire, UK, 1992; pp. 20–29. [Google Scholar]
- Tang, Y.; Hartt, W.; Granata, R.; Yu, H.; Farooq, M.U. Degradation of carbon/vinyl ester composites under cathodic polarization in seawater. J. Compos. Mater. 2012, 46, 3115–3120. [Google Scholar] [CrossRef]
- Shin, E.E.; Morgan, R.J.; Zhou, J.M.; Lincoln, J.; Jurek, R.; Curliss, D.B. Hygrothermal durability and thermal aging behavior prediction of high-temperature polymer-matrix composites and their resins. J. Thermoplast. Compos. Mater. 2000, 13, 40–57. [Google Scholar] [CrossRef]
- Shin, E.E.; Morgan, R.J.; Zhou, J. Hydrolytic Degradation Mechanisms and Kinetics of Polyimides for Advanced Composites. In Proceedings of the SAMPE 2000, Long Beach, CA, USA, 21–25 May 2000. [Google Scholar]
- Boyd, J.; Chang, G.; Webb, W.; Speak, S.; Gerth, D. Galvanic Corrosion Effects on Carbon Fiber Composites. In Proceedings of the 36th International SAMPE Symposium and Exhibition, San Diego, CA, USA, 15–18 April 1991; p. 1217. [Google Scholar]
- Cochran, R.C.; Trabocco, R.E.; Boodey, J.; Thomson, J.; Donnellan, T.M. Degradation of imide based composites. In Proceedings of the 36th International SAMPE Symposium and Exhibition, San Diego, CA, USA, 15–18 April 1991; p. 1273. [Google Scholar]
- Faudree, M.C. Relationship of Graphite-Polyimide Composites to Galvanic Processes. In Proceedings of the 36th International SAMPE Symposium and Exhibition, San Diego, CA, USA, 15–18 April 1991; p. 1288. [Google Scholar]
- Sloan, F.E.; Talbot, J.B. Corrosion of Graphite-Fibre-Reinforced Composites II-Anodic Polarization Damage. Corrosion 1992, 48, 1020–1026. [Google Scholar] [CrossRef]
- Stafford, G.R.; Cahen, G.L.; Stoner, G.E. Graphite Fibre-Polymer Matrix Composites as Electrolysis Electrodes. J. Electrochem. Soc. 1991, 138, 425–430. [Google Scholar] [CrossRef]
- Pittman, C.U.; Jiang, W.; Yue, Z.R.; Gardner, S.; Wang, L.; Toghiani, H.; y Leon, C.L. Surface properties of electrochemically oxidized carbon fibres. Carbon 1999, 37, 1797–1807. [Google Scholar] [CrossRef]
- Bismarck, A.; Kumru, M.E.; Springer, J.; Simitzis, J. Surface properties of PAN-based carbon fibres tuned by anodic oxidation in different alkaline electrolyte systems. Appl. Surf. Sci. 1999, 143, 45–55. [Google Scholar] [CrossRef]
- Taylor, S.R. A nondestructive electrochemical method to detect and quantify graphite fibre/polymer matrix disbondment in aqueous and cathodically polarized conditions. Compos. Interfaces 1994, 2, 403–417. [Google Scholar]
- Ofoegbu, S.U.; Zheludkevich, M.L.; Kallip, S.; Ferreira, M.G.S. Electrochemical studies of carbon fibre reinforced plastics in chloride media containing selected inhibitors. In Proceedings of the 19th SPE Meeting, Aveiro, Portugal, 30 June–2 July 2014. [Google Scholar]
- Bokobza, L. Multiwall carbon nanotube elastomeric composites: A review. Polymer 2007, 48, 4907–4920. [Google Scholar] [CrossRef] [Green Version]
- De Levie, R. On porous electrodes in electrolyte solutions: I. Capacitance effects. Electrochim. Acta 1963, 8, 751–780. [Google Scholar] [CrossRef]
- De Levie, R. On porous electrodes in electrolyte solutions—IV. Electrochim. Acta 1964, 9, 1231–1245. [Google Scholar] [CrossRef]
- De Levie, R. The influence of surface roughness of solid electrodes on electrochemical measurements. Electrochim. Acta 1965, 10, 113–130. [Google Scholar] [CrossRef]
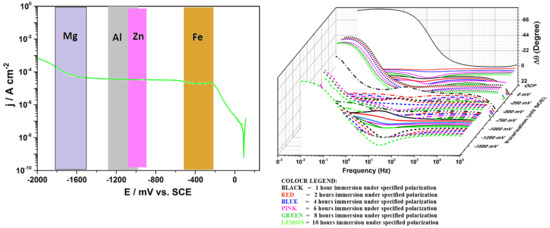
- Ofoegbu, S.U.; Ferreira, M.G.S.; Zheludkevich, M.L. On the Use of Delta phase angle (∆θ) to Monitor Damage in Carbon Fibre Reinforced Polymer Composites. 2019; submitted. [Google Scholar]
- Ofoegbu, S.U.; Quevedo, M.C.; Bastos, A.C.; Ferreira, M.G.S.; Zheludkevich, M.L. Electrochemical Response of Carbon Fibre Reinforced Polymer (CFRP) in Quiescent Near Neutral Aqueous Chloride Media. 2019; submitted. [Google Scholar]
- Ofoegbu, S.U.; Yasakau, K.; Kallip, S.; Nogueira, H.I.; Ferreira, M.G.S.; Zheludkevich, M.L. Modification of carbon fibre reinforced polymer (CFRP) surface with sodium dodecyl sulphate for mitigation of cathodic activity. Appl. Surf. Sci. 2019, 478, 924–936. [Google Scholar] [CrossRef]
- Glass, R.C.; Taylor, S.R.; Cahen Jr, G.L.; Stoner, G.E. Electrochemical impedance spectroscopy as a method to nondestructively monitor simulated in-service damage in a carbon fibre reinforced plastic. J. Nondestruct. Eval. 1987, 6, 181–188. [Google Scholar] [CrossRef]
- Fazzino, P.; Reifsnider, K. Electrochemical impedance spectroscopy detection of damage in out of plane fatigued fibre reinforced composite materials. Appl. Compos. Mater. 2008, 15, 127–138. [Google Scholar] [CrossRef]
- Fazzino, P.D.; Reifsnider, K.L.; Majumdar, P. Impedance spectroscopy for progressive damage analysis in woven composites. Compos. Sci. Technol. 2009, 69, 2008–2014. [Google Scholar] [CrossRef]
- Torrents, J.M.; Mason, T.O.; Peled, A.; Shah, S.P.; Garboczi, E.J. Analysis of the impedance spectra of short conductive fibre-reinforced composites. J. Mater. Sci. 2001, 36, 4003–4012. [Google Scholar] [CrossRef]
- Radovic, L.R.; Moreno-Castilla, C.; Rivera-Utrilla, J. Carbon Materials as Adsorbents in Aqueous Solutions; Marcel Dekker Inc.: New York, NY, USA, 2001; Volume 27, pp. 227–406. [Google Scholar]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 2004, 42, 83–94. [Google Scholar] [CrossRef] [Green Version]







| Thermosets | Thermoplastics | |||
|---|---|---|---|---|
| Forms Cross-Linked Networks in Polymerization Curing by Heating | No Chemical Change | |||
| Epoxies | Phenolics | Polyester | Polyimides | PPS, PEEK |
|
|
|
|
|
|
|
| ||
|
|
| ||
|
|
|
| |
|
|
|
| |
|
|
|
| |
| Composite System | Filler Used | Filler wt % | Electrical Conductivity (S/cm) | Reference |
|---|---|---|---|---|
| CNF-Epoxy matrix | CNF | 0.1–1 | 2 ×10−6 to 4 ×10−3 | Bal (2010) Ref. [232] |
| CNT-polystyrene | MWCNT | 0.5–4 | (1 × 10−10 to 1 S/cm) (1 × 10−8 to 1 S/m)* | Cipriano et al. (2008) Ref. [264] |
| CNF-polystyrene | CNF | 3–15 | 1 × 10−10 to 10−2 S/cm (an) 1 × 10−10 to 10−5 S/cm (un-an) (1 × 10−8 to near 1 S/m)* | Cipriano et al. (2008) Ref. [264] |
| CNT-polycarbonate | MWCNT | 0.75–3 | 1 × 10−14 to > 10−2 S/cm (at 1 Hz) | Pötschke et al. (2004a) Ref. [266] |
| CNT-polycarbonate | MWCNT | 0.875 | ≈ 10−3 S/cm (post annealing at 230 °C) | Alig et al. (2007) Ref. [268] |
| CNT-polycarbonate | SWCNT MWCNT | 1 × 10−13 to > 10−3 S/cm (at 1 Hz during mixing) | Pötschke et al. (2004b) Ref. [269] | |
| CNT-polycarbonate | MWCNT | 0–5 | 1 × 10−16 to ≈10−2 S/cm | Pötschke et al. (2003) Ref. [270] |
| CNT-epoxy CNF-polypropylene CNF-polyethylene | SWCNT CNF CNF | 0–40 0–40 0–40 | 1 ×10−16 to ≈ 10−7 S/cm 1 × 10−17 to ≈ 10−7 S/cm 1 × 10−17 to ≈ 10−7 S/cm | Lozano et al. (2001) Ref. [264] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ofoegbu, S.U.; Ferreira, M.G.S.; Zheludkevich, M.L. Galvanically Stimulated Degradation of Carbon-Fiber Reinforced Polymer Composites: A Critical Review. Materials 2019, 12, 651. https://doi.org/10.3390/ma12040651
Ofoegbu SU, Ferreira MGS, Zheludkevich ML. Galvanically Stimulated Degradation of Carbon-Fiber Reinforced Polymer Composites: A Critical Review. Materials. 2019; 12(4):651. https://doi.org/10.3390/ma12040651
Chicago/Turabian StyleOfoegbu, Stanley Udochukwu, Mário G.S. Ferreira, and Mikhail L. Zheludkevich. 2019. "Galvanically Stimulated Degradation of Carbon-Fiber Reinforced Polymer Composites: A Critical Review" Materials 12, no. 4: 651. https://doi.org/10.3390/ma12040651